HIV/AIDS
| HIV/AIDS | |
|---|---|
| Synonyms | HIV disease, HIV infection[1][2][3] |
 | |
| The red ribbon is a symbol for solidarity with HIV-positive people and those living with AIDS.[4] | |
| Classification and external resources | |
| Specialty | Infectious disease |
| ICD-10 | B20 – B24 |
| ICD-9-CM | 042-044 |
| OMIM | 609423 |
| DiseasesDB | 5938 |
| MedlinePlus | 000594 |
| eMedicine | emerg/253 |
| Patient UK | HIV/AIDS |
| MeSH | D000163 |
Human immunodeficiency virus infection and acquired immune deficiency syndrome (HIV/AIDS) is a spectrum of conditions caused by infection with the human immunodeficiency virus (HIV).[5][6][7] Following initial infection, a person may not notice any symptoms or may experience a brief period of influenza-like illness.[8] Typically, this is followed by a prolonged period with no symptoms.[9] As the infection progresses, it interferes more with the immune system, increasing the risk of common infections like tuberculosis, as well as other opportunistic infections, and tumors that rarely affect people who have working immune systems.[8] These late symptoms of infection are referred to as AIDS.[9] This stage is often also associated with weight loss.[9]
HIV is spread primarily by unprotected sex (including anal and oral sex), contaminated blood transfusions, hypodermic needles, and from mother to child during pregnancy, delivery, or breastfeeding.[10] Some bodily fluids, such as saliva and tears, do not transmit HIV.[11] Methods of prevention include safe sex, needle exchange programs, treating those who are infected, and male circumcision.[8] Disease in a baby can often be prevented by giving both the mother and child antiretroviral medication.[8] There is no cure or vaccine; however, antiretroviral treatment can slow the course of the disease and may lead to a near-normal life expectancy.[9][12] Treatment is recommended as soon as the diagnosis is made.[13] Without treatment, the average survival time after infection is 11 years.[14]
In 2015 about 36.7 million people were living with HIV and it resulted in 1.1 million deaths.[8] Most of those infected live in sub-Saharan Africa.[8] Between its discovery and 2014 AIDS has caused an estimated 39 million deaths worldwide.[15] HIV/AIDS is considered a pandemic—a disease outbreak which is present over a large area and is actively spreading.[16] HIV is believed to have originated in west-central Africa during the late 19th or early 20th century.[17] AIDS was first recognized by the United States Centers for Disease Control and Prevention (CDC) in 1981 and its cause—HIV infection—was identified in the early part of the decade.[18]
HIV/AIDS has had a great impact on society, both as an illness and as a source of discrimination.[19] The disease also has large economic impacts.[19] There are many misconceptions about HIV/AIDS such as the belief that it can be transmitted by casual non-sexual contact.[20] The disease has become subject to many controversies involving religion including the Catholic Church's decision not to support condom use as prevention.[21] It has attracted international medical and political attention as well as large-scale funding since it was identified in the 1980s.[22]

Signs and symptoms
There are three main stages of HIV infection: acute infection, clinical latency and AIDS.[1]
Acute infection
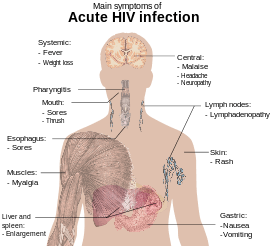
The initial period following the contraction of HIV is called acute HIV, primary HIV or acute retroviral syndrome.[2][23] Many individuals develop an influenza-like illness or a mononucleosis-like illness 2–4 weeks post exposure while others have no significant symptoms.[24][25] Symptoms occur in 40–90% of cases and most commonly include fever, large tender lymph nodes, throat inflammation, a rash, headache, and/or sores of the mouth and genitals.[23][25] The rash, which occurs in 20–50% of cases, presents itself on the trunk and is maculopapular, classically.[26] Some people also develop opportunistic infections at this stage.[23] Gastrointestinal symptoms such as nausea, vomiting or diarrhea may occur, as may neurological symptoms of peripheral neuropathy or Guillain–Barré syndrome.[25] The duration of the symptoms varies, but is usually one or two weeks.[25]
Due to their nonspecific character, these symptoms are not often recognized as signs of HIV infection. Even cases that do get seen by a family doctor or a hospital are often misdiagnosed as one of the many common infectious diseases with overlapping symptoms. Thus, it is recommended that HIV be considered in people presenting an unexplained fever who may have risk factors for the infection.[25]
Clinical latency
The initial symptoms are followed by a stage called clinical latency, asymptomatic HIV, or chronic HIV.[1] Without treatment, this second stage of the natural history of HIV infection can last from about three years[27] to over 20 years[28] (on average, about eight years).[29] While typically there are few or no symptoms at first, near the end of this stage many people experience fever, weight loss, gastrointestinal problems and muscle pains.[1] Between 50 and 70% of people also develop persistent generalized lymphadenopathy, characterized by unexplained, non-painful enlargement of more than one group of lymph nodes (other than in the groin) for over three to six months.[2]
Although most HIV-1 infected individuals have a detectable viral load and in the absence of treatment will eventually progress to AIDS, a small proportion (about 5%) retain high levels of CD4+ T cells (T helper cells) without antiretroviral therapy for more than 5 years.[25][30] These individuals are classified as HIV controllers or long-term nonprogressors (LTNP).[30] Another group consists of those who maintain a low or undetectable viral load without anti-retroviral treatment, known as "elite controllers" or "elite suppressors". They represent approximately 1 in 300 infected persons.[31]
Acquired immunodeficiency syndrome
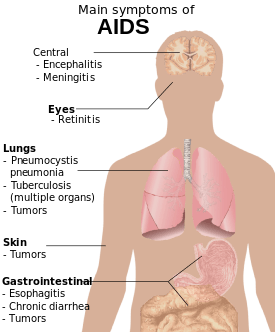
Acquired immunodeficiency syndrome (AIDS) is defined in terms of either a CD4+ T cell count below 200 cells per µL or the occurrence of specific diseases in association with an HIV infection.[25] In the absence of specific treatment, around half of people infected with HIV develop AIDS within ten years.[25] The most common initial conditions that alert to the presence of AIDS are pneumocystis pneumonia (40%), cachexia in the form of HIV wasting syndrome (20%), and esophageal candidiasis.[25] Other common signs include recurring respiratory tract infections.[25]
Opportunistic infections may be caused by bacteria, viruses, fungi, and parasites that are normally controlled by the immune system.[32] Which infections occur depends partly on what organisms are common in the person's environment.[25] These infections may affect nearly every organ system.[33]
People with AIDS have an increased risk of developing various viral-induced cancers, including Kaposi's sarcoma, Burkitt's lymphoma, primary central nervous system lymphoma, and cervical cancer.[26] Kaposi's sarcoma is the most common cancer occurring in 10 to 20% of people with HIV.[34] The second most common cancer is lymphoma, which is the cause of death of nearly 16% of people with AIDS and is the initial sign of AIDS in 3 to 4%.[34] Both these cancers are associated with human herpesvirus 8.[34] Cervical cancer occurs more frequently in those with AIDS because of its association with human papillomavirus (HPV).[34] Conjunctival cancer (of the layer that lines the inner part of eyelids and the white part of the eye) is also more common in those with HIV.[35]
Additionally, people with AIDS frequently have systemic symptoms such as prolonged fevers, sweats (particularly at night), swollen lymph nodes, chills, weakness, and unintended weight loss.[36] Diarrhea is another common symptom, present in about 90% of people with AIDS.[37] They can also be affected by diverse psychiatric and neurological symptoms independent of opportunistic infections and cancers.[38]
Transmission
| Exposure route | Chance of infection | |||
|---|---|---|---|---|
| Blood transfusion | 90%[39] | |||
| Childbirth (to child) | 25%[40] | |||
| Needle-sharing injection drug use | 0.67%[39] | |||
| Percutaneous needle stick | 0.30%[41] | |||
| Receptive anal intercourse* | 0.04–3.0%[42] | |||
| Insertive anal intercourse* | 0.03%[43] | |||
| Receptive penile-vaginal intercourse* | 0.05–0.30%[42][44] | |||
| Insertive penile-vaginal intercourse* | 0.01–0.38%[42][44] | |||
| Receptive oral intercourse*§ | 0–0.04%[42] | |||
| Insertive oral intercourse*§ | 0–0.005%[45] | |||
| * assuming no condom use § source refers to oral intercourse performed on a man | ||||
HIV is transmitted by three main routes: sexual contact, significant exposure to infected body fluids or tissues, and from mother to child during pregnancy, delivery, or breastfeeding (known as vertical transmission).[10] There is no risk of acquiring HIV if exposed to feces, nasal secretions, saliva, sputum, sweat, tears, urine, or vomit unless these are contaminated with blood.[46] It is possible to be co-infected by more than one strain of HIV—a condition known as HIV superinfection.[47]
Sexual
The most frequent mode of transmission of HIV is through sexual contact with an infected person.[10] The majority of all transmissions worldwide occur through heterosexual contacts (i.e. sexual contacts between people of the opposite sex);[10] however, the pattern of transmission varies significantly among countries. In the United States, as of 2010, most transmission occurred in men who had sex with men, with this population accounting for 65% of all new cases.[48]
With regard to unprotected heterosexual contacts, estimates of the risk of HIV transmission per sexual act appear to be four to ten times higher in low-income countries than in high-income countries.[49] In low-income countries, the risk of female-to-male transmission is estimated as 0.38% per act, and of male-to-female transmission as 0.30% per act; the equivalent estimates for high-income countries are 0.04% per act for female-to-male transmission, and 0.08% per act for male-to-female transmission.[49] The risk of transmission from anal intercourse is especially high, estimated as 1.4–1.7% per act in both heterosexual and homosexual contacts.[49][50] While the risk of transmission from oral sex is relatively low, it is still present.[51] The risk from receiving oral sex has been described as "nearly nil";[52] however, a few cases have been reported.[53] The per-act risk is estimated at 0–0.04% for receptive oral intercourse.[54] In settings involving prostitution in low income countries, risk of female-to-male transmission has been estimated as 2.4% per act and male-to-female transmission as 0.05% per act.[49]
Risk of transmission increases in the presence of many sexually transmitted infections[55] and genital ulcers.[49] Genital ulcers appear to increase the risk approximately fivefold.[49] Other sexually transmitted infections, such as gonorrhea, chlamydia, trichomoniasis, and bacterial vaginosis, are associated with somewhat smaller increases in risk of transmission.[54]
The viral load of an infected person is an important risk factor in both sexual and mother-to-child transmission.[56] During the first 2.5 months of an HIV infection a person's infectiousness is twelve times higher due to this high viral load.[54] If the person is in the late stages of infection, rates of transmission are approximately eightfold greater.[49]
Commercial sex workers (including those in pornography) have an increased rate of HIV.[57][58] Rough sex can be a factor associated with an increased risk of transmission.[59] Sexual assault is also believed to carry an increased risk of HIV transmission as condoms are rarely worn, physical trauma to the vagina or rectum is likely, and there may be a greater risk of concurrent sexually transmitted infections.[60]
Body fluids

The second most frequent mode of HIV transmission is via blood and blood products.[10] Blood-borne transmission can be through needle-sharing during intravenous drug use, needle stick injury, transfusion of contaminated blood or blood product, or medical injections with unsterilized equipment. The risk from sharing a needle during drug injection is between 0.63 and 2.4% per act, with an average of 0.8%.[61] The risk of acquiring HIV from a needle stick from an HIV-infected person is estimated as 0.3% (about 1 in 333) per act and the risk following mucous membrane exposure to infected blood as 0.09% (about 1 in 1000) per act.[46] In the United States intravenous drug users made up 12% of all new cases of HIV in 2009,[62] and in some areas more than 80% of people who inject drugs are HIV positive.[10]
HIV is transmitted in about 93% of blood transfusions using infected blood.[61] In developed countries the risk of acquiring HIV from a blood transfusion is extremely low (less than one in half a million) where improved donor selection and HIV screening is performed;[10] for example, in the UK the risk is reported at one in five million[63] and in the United States it was one in 1.5 million in 2008.[64] In low income countries, only half of transfusions may be appropriately screened (as of 2008),[65] and it is estimated that up to 15% of HIV infections in these areas come from transfusion of infected blood and blood products, representing between 5% and 10% of global infections.[10][66] Although rare because of screening, it is possible to acquire HIV from organ and tissue transplantation.[67]
Unsafe medical injections play a significant role in HIV spread in sub-Saharan Africa. In 2007, between 12 and 17% of infections in this region were attributed to medical syringe use.[68] The World Health Organization estimates the risk of transmission as a result of a medical injection in Africa at 1.2%.[68] Significant risks are also associated with invasive procedures, assisted delivery, and dental care in this area of the world.[68]
People giving or receiving tattoos, piercings, and scarification are theoretically at risk of infection but no confirmed cases have been documented.[69] It is not possible for mosquitoes or other insects to transmit HIV.[70]
Mother-to-child
HIV can be transmitted from mother to child during pregnancy, during delivery, or through breast milk resulting in infection in the baby.[71][72] This is the third most common way in which HIV is transmitted globally.[10] In the absence of treatment, the risk of transmission before or during birth is around 20% and in those who also breastfeed 35%.[71] As of 2008, vertical transmission accounted for about 90% of cases of HIV in children.[71] With appropriate treatment the risk of mother-to-child infection can be reduced to about 1%.[71] Preventive treatment involves the mother taking antiretrovirals during pregnancy and delivery, an elective caesarean section, avoiding breastfeeding, and administering antiretroviral drugs to the newborn.[73] Antiretrovirals when taken by either the mother or the infant decrease the risk of transmission in those who do breastfeed.[74] Many of these measures are however not available in the developing world.[73] If blood contaminates food during pre-chewing it may pose a risk of transmission.[69]
Virology
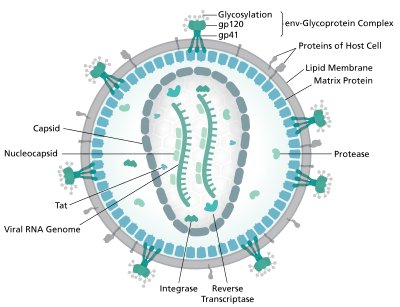

HIV is the cause of the spectrum of disease known as HIV/AIDS. HIV is a retrovirus that primarily infects components of the human immune system such as CD4+ T cells, macrophages and dendritic cells. It directly and indirectly destroys CD4+ T cells.[75]
HIV is a member of the genus Lentivirus,[76] part of the family Retroviridae.[77] Lentiviruses share many morphological and biological characteristics. Many species of mammals are infected by lentiviruses, which are characteristically responsible for long-duration illnesses with a long incubation period.[78] Lentiviruses are transmitted as single-stranded, positive-sense, enveloped RNA viruses. Upon entry into the target cell, the viral RNA genome is converted (reverse transcribed) into double-stranded DNA by a virally encoded reverse transcriptase that is transported along with the viral genome in the virus particle. The resulting viral DNA is then imported into the cell nucleus and integrated into the cellular DNA by a virally encoded integrase and host co-factors.[79] Once integrated, the virus may become latent, allowing the virus and its host cell to avoid detection by the immune system.[80] Alternatively, the virus may be transcribed, producing new RNA genomes and viral proteins that are packaged and released from the cell as new virus particles that begin the replication cycle anew.[81]
HIV is now known to spread between CD4+ T cells by two parallel routes: cell-free spread and cell-to-cell spread, i.e. it employs hybrid spreading mechanisms.[82] In the cell-free spread, virus particles bud from an infected T cell, enter the blood/extracellular fluid and then infect another T cell following a chance encounter.[82] HIV can also disseminate by direct transmission from one cell to another by a process of cell-to-cell spread.[83][84] The hybrid spreading mechanisms of HIV contribute to the virus's ongoing replication against antiretroviral therapies.[82][85]
Two types of HIV have been characterized: HIV-1 and HIV-2. HIV-1 is the virus that was originally discovered (and initially referred to also as LAV or HTLV-III). It is more virulent, more infective,[86] and is the cause of the majority of HIV infections globally. The lower infectivity of HIV-2 as compared with HIV-1 implies that fewer people exposed to HIV-2 will be infected per exposure. Because of its relatively poor capacity for transmission, HIV-2 is largely confined to West Africa.[87]
Pathophysiology

After the virus enters the body there is a period of rapid viral replication, leading to an abundance of virus in the peripheral blood. During primary infection, the level of HIV may reach several million virus particles per milliliter of blood.[88] This response is accompanied by a marked drop in the number of circulating CD4+ T cells. The acute viremia is almost invariably associated with activation of CD8+ T cells, which kill HIV-infected cells, and subsequently with antibody production, or seroconversion. The CD8+ T cell response is thought to be important in controlling virus levels, which peak and then decline, as the CD4+ T cell counts recover. A good CD8+ T cell response has been linked to slower disease progression and a better prognosis, though it does not eliminate the virus.[89]
Ultimately, HIV causes AIDS by depleting CD4+ T cells. This weakens the immune system and allows opportunistic infections. T cells are essential to the immune response and without them, the body cannot fight infections or kill cancerous cells. The mechanism of CD4+ T cell depletion differs in the acute and chronic phases.[90] During the acute phase, HIV-induced cell lysis and killing of infected cells by cytotoxic T cells accounts for CD4+ T cell depletion, although apoptosis may also be a factor. During the chronic phase, the consequences of generalized immune activation coupled with the gradual loss of the ability of the immune system to generate new T cells appear to account for the slow decline in CD4+ T cell numbers.[91]
Although the symptoms of immune deficiency characteristic of AIDS do not appear for years after a person is infected, the bulk of CD4+ T cell loss occurs during the first weeks of infection, especially in the intestinal mucosa, which harbors the majority of the lymphocytes found in the body.[92] The reason for the preferential loss of mucosal CD4+ T cells is that the majority of mucosal CD4+ T cells express the CCR5 protein which HIV uses as a co-receptor to gain access to the cells, whereas only a small fraction of CD4+ T cells in the bloodstream do so.[93] A specific genetic change that alters the CCR5 protein when present in both chromosomes very effectively prevents HIV-1 infection.[94]
HIV seeks out and destroys CCR5 expressing CD4+ T cells during acute infection.[95] A vigorous immune response eventually controls the infection and initiates the clinically latent phase. CD4+ T cells in mucosal tissues remain particularly affected.[95] Continuous HIV replication causes a state of generalized immune activation persisting throughout the chronic phase.[96] Immune activation, which is reflected by the increased activation state of immune cells and release of pro-inflammatory cytokines, results from the activity of several HIV gene products and the immune response to ongoing HIV replication. It is also linked to the breakdown of the immune surveillance system of the gastrointestinal mucosal barrier caused by the depletion of mucosal CD4+ T cells during the acute phase of disease.[97]
Diagnosis
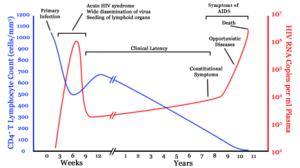
HIV/AIDS is diagnosed via laboratory testing and then staged based on the presence of certain signs or symptoms.[23] HIV screening is recommended by the United States Preventive Services Task Force for all people 15 years to 65 years of age including all pregnant women.[98] Additionally, testing is recommended for those at high risk, which includes anyone diagnosed with a sexually transmitted illness.[26] In many areas of the world, a third of HIV carriers only discover they are infected at an advanced stage of the disease when AIDS or severe immunodeficiency has become apparent.[26]
HIV testing
Most people infected with HIV develop specific antibodies (i.e. seroconvert) within three to twelve weeks of the initial infection.[25] Diagnosis of primary HIV before seroconversion is done by measuring HIV-RNA or p24 antigen.[25] Positive results obtained by antibody or PCR testing are confirmed either by a different antibody or by PCR.[23]
Antibody tests in children younger than 18 months are typically inaccurate due to the continued presence of maternal antibodies.[99] Thus HIV infection can only be diagnosed by PCR testing for HIV RNA or DNA, or via testing for the p24 antigen.[23] Much of the world lacks access to reliable PCR testing and many places simply wait until either symptoms develop or the child is old enough for accurate antibody testing.[99] In sub-Saharan Africa as of 2007–2009 between 30 and 70% of the population were aware of their HIV status.[100] In 2009, between 3.6 and 42% of men and women in Sub-Saharan countries were tested[100] which represented a significant increase compared to previous years.[100]
Classifications
Two main clinical staging systems are used to classify HIV and HIV-related disease for surveillance purposes: the WHO disease staging system for HIV infection and disease,[23] and the CDC classification system for HIV infection.[101] The CDC's classification system is more frequently adopted in developed countries. Since the WHO's staging system does not require laboratory tests, it is suited to the resource-restricted conditions encountered in developing countries, where it can also be used to help guide clinical management. Despite their differences, the two systems allow comparison for statistical purposes.[2][23][101]
The World Health Organization first proposed a definition for AIDS in 1986.[23] Since then, the WHO classification has been updated and expanded several times, with the most recent version being published in 2007.[23] The WHO system uses the following categories:
- Primary HIV infection: May be either asymptomatic or associated with acute retroviral syndrome.[23]
- Stage I: HIV infection is asymptomatic with a CD4+ T cell count (also known as CD4 count) greater than 500 per microlitre (µl or cubic mm) of blood.[23] May include generalized lymph node enlargement.[23]
- Stage II: Mild symptoms which may include minor mucocutaneous manifestations and recurrent upper respiratory tract infections. A CD4 count of less than 500/µl.[23]
- Stage III: Advanced symptoms which may include unexplained chronic diarrhea for longer than a month, severe bacterial infections including tuberculosis of the lung, and a CD4 count of less than 350/µl.[23]
- Stage IV or AIDS: severe symptoms which include toxoplasmosis of the brain, candidiasis of the esophagus, trachea, bronchi or lungs and Kaposi's sarcoma. A CD4 count of less than 200/µl.[23]
The United States Center for Disease Control and Prevention also created a classification system for HIV, and updated it in 2008 and 2014.[101][102] This system classifies HIV infections based on CD4 count and clinical symptoms, and describes the infection in five groups.[102] In those greater than six years of age it is:[102]
- Stage 0: the time between a negative or indeterminate HIV test followed less than 180 days by a positive test
- Stage 1: CD4 count ≥ 500 cells/µl and no AIDS defining conditions
- Stage 2: CD4 count 200 to 500 cells/µl and no AIDS defining conditions
- Stage 3: CD4 count ≤ 200 cells/µl or AIDS defining conditions
- Unknown: if insufficient information is available to make any of the above classifications
For surveillance purposes, the AIDS diagnosis still stands even if, after treatment, the CD4+ T cell count rises to above 200 per µL of blood or other AIDS-defining illnesses are cured.[2]
Prevention

Sexual contact
Consistent condom use reduces the risk of HIV transmission by approximately 80% over the long term.[103] When condoms are used consistently by a couple in which one person is infected, the rate of HIV infection is less than 1% per year.[104] There is some evidence to suggest that female condoms may provide an equivalent level of protection.[105] Application of a vaginal gel containing tenofovir (a reverse transcriptase inhibitor) immediately before sex seems to reduce infection rates by approximately 40% among African women.[106] By contrast, use of the spermicide nonoxynol-9 may increase the risk of transmission due to its tendency to cause vaginal and rectal irritation.[107]
Circumcision in Sub-Saharan Africa "reduces the acquisition of HIV by heterosexual men by between 38% and 66% over 24 months".[108] Due to these studies, both the World Health Organization and UNAIDS recommended male circumcision as a method of preventing female-to-male HIV transmission in 2007 in areas with a high rates of HIV.[109] However, whether it protects against male-to-female transmission is disputed,[110][111] and whether it is of benefit in developed countries and among men who have sex with men is undetermined.[112][113][114] The International Antiviral Society, however, does recommend for all sexually active heterosexual males and that it be discussed as an option with men who have sex with men.[115] Some experts fear that a lower perception of vulnerability among circumcised men may cause more sexual risk-taking behavior, thus negating its preventive effects.[116]
Programs encouraging sexual abstinence do not appear to affect subsequent HIV risk.[117] Evidence of any benefit from peer education is equally poor.[118] Comprehensive sexual education provided at school may decrease high risk behavior.[119] A substantial minority of young people continues to engage in high-risk practices despite knowing about HIV/AIDS, underestimating their own risk of becoming infected with HIV.[120] Voluntary counseling and testing people for HIV does not affect risky behavior in those who test negative but does increase condom use in those who test positive.[121] It is not known whether treating other sexually transmitted infections is effective in preventing HIV.[55]
Pre-exposure
Antiretroviral treatment among people with HIV whose CD4 count ≤ 550 cells/µL is a very effective way to prevent HIV infection of their partner (a strategy known as treatment as prevention, or TASP).[122] TASP is associated with a 10 to 20 fold reduction in transmission risk.[122][123] Pre-exposure prophylaxis (PrEP) with a daily dose of the medications tenofovir, with or without emtricitabine, is effective in a number of groups including men who have sex with men, couples where one is HIV positive, and young heterosexuals in Africa.[106] It may also be effective in intravenous drug users with a study finding a decrease in risk of 0.7 to 0.4 per 100 person years.[124]
Universal precautions within the health care environment are believed to be effective in decreasing the risk of HIV.[125] Intravenous drug use is an important risk factor and harm reduction strategies such as needle-exchange programs and opioid substitution therapy appear effective in decreasing this risk.[126][127]
Post-exposure
A course of antiretrovirals administered within 48 to 72 hours after exposure to HIV-positive blood or genital secretions is referred to as post-exposure prophylaxis (PEP).[128] The use of the single agent zidovudine reduces the risk of a HIV infection five-fold following a needle-stick injury.[128] As of 2013, the prevention regimen recommended in the United States consists of three medications—tenofovir, emtricitabine and raltegravir—as this may reduce the risk further.[129]
PEP treatment is recommended after a sexual assault when the perpetrator is known to be HIV positive, but is controversial when their HIV status is unknown.[130] The duration of treatment is usually four weeks[131] and is frequently associated with adverse effects—where zidovudine is used, about 70% of cases result in adverse effects such as nausea (24%), fatigue (22%), emotional distress (13%) and headaches (9%).[46]
Mother-to-child
Programs to prevent the vertical transmission of HIV (from mothers to children) can reduce rates of transmission by 92–99%.[71][126] This primarily involves the use of a combination of antiviral medications during pregnancy and after birth in the infant and potentially includes bottle feeding rather than breastfeeding.[71][132] If replacement feeding is acceptable, feasible, affordable, sustainable, and safe, mothers should avoid breastfeeding their infants; however exclusive breastfeeding is recommended during the first months of life if this is not the case.[133] If exclusive breastfeeding is carried out, the provision of extended antiretroviral prophylaxis to the infant decreases the risk of transmission.[134] In 2015, Cuba became the first country in the world to eradicate mother-to-child transmission of HIV.[135]
Vaccination
Currently, there is no licensed vaccine for HIV or AIDS.[12] The most effective vaccine trial to date, RV 144, was published in 2009 and found a partial reduction in the risk of transmission of roughly 30%, stimulating some hope in the research community of developing a truly effective vaccine.[136] Further trials of the RV 144 vaccine are ongoing.[137][138]
Treatment
There is currently no cure or effective HIV vaccine. Treatment consists of highly active antiretroviral therapy (HAART) which slows progression of the disease.[139] As of 2010 more than 6.6 million people were taking them in low and middle income countries.[140] Treatment also includes preventive and active treatment of opportunistic infections.
Antiviral therapy

Current HAART options are combinations (or "cocktails") consisting of at least three medications belonging to at least two types, or "classes," of antiretroviral agents.[141] Initially treatment is typically a non-nucleoside reverse transcriptase inhibitor (NNRTI) plus two nucleoside analog reverse transcriptase inhibitors (NRTIs).[142] Typical NRTIs include: zidovudine (AZT) or tenofovir (TDF) and lamivudine (3TC) or emtricitabine (FTC).[142] Combinations of agents which include protease inhibitors (PI) are used if the above regimen loses effectiveness.[141]
The World Health Organization and United States recommends antiretrovirals in people of all ages including pregnant women as soon as the diagnosis is made regardless of CD4 count.[13][115][143] Once treatment is begun it is recommended that it is continued without breaks or "holidays".[26] Many people are diagnosed only after treatment ideally should have begun.[26] The desired outcome of treatment is a long term plasma HIV-RNA count below 50 copies/mL.[26] Levels to determine if treatment is effective are initially recommended after four weeks and once levels fall below 50 copies/mL checks every three to six months are typically adequate.[26] Inadequate control is deemed to be greater than 400 copies/mL.[26] Based on these criteria treatment is effective in more than 95% of people during the first year.[26]
Benefits of treatment include a decreased risk of progression to AIDS and a decreased risk of death.[144] In the developing world treatment also improves physical and mental health.[145] With treatment there is a 70% reduced risk of acquiring tuberculosis.[141] Additional benefits include a decreased risk of transmission of the disease to sexual partners and a decrease in mother-to-child transmission.[141] The effectiveness of treatment depends to a large part on compliance.[26] Reasons for non-adherence include poor access to medical care,[146] inadequate social supports, mental illness and drug abuse.[147] The complexity of treatment regimens (due to pill numbers and dosing frequency) and adverse effects may reduce adherence.[148] Even though cost is an important issue with some medications,[149] 47% of those who needed them were taking them in low and middle income countries as of 2010[140] and the rate of adherence is similar in low-income and high-income countries.[150]
Specific adverse events are related to the antiretroviral agent taken.[151] Some relatively common adverse events include: lipodystrophy syndrome, dyslipidemia, and diabetes mellitus, especially with protease inhibitors.[2] Other common symptoms include diarrhea,[151][152] and an increased risk of cardiovascular disease.[153] Newer recommended treatments are associated with fewer adverse effects.[26] Certain medications may be associated with birth defects and therefore may be unsuitable for women hoping to have children.[26]
Treatment recommendations for children are somewhat different from those for adults. The World Health Organization recommends treating all children less than 5 years of age; children above 5 are treated like adults.[154] The United States guidelines recommend treating all children less than 12 months of age and all those with HIV RNA counts greater than 100,000 copies/mL between one year and five years of age.[155]
Opportunistic infections
Measures to prevent opportunistic infections are effective in many people with HIV/AIDS. In addition to improving current disease, treatment with antiretrovirals reduces the risk of developing additional opportunistic infections.[151] Adults and adolescents who are living with HIV (even on anti-retroviral therapy) with no evidence of active tuberculosis in settings with high tuberculosis burden should receive isoniazid preventive therapy (IPT), the tuberculin skin test can be used to help decide if IPT is needed.[156] Vaccination against hepatitis A and B is advised for all people at risk of HIV before they become infected; however it may also be given after infection.[157] Trimethoprim/sulfamethoxazole prophylaxis between four and six weeks of age and ceasing breastfeeding in infants born to HIV positive mothers is recommended in resource limited settings.[158] It is also recommended to prevent PCP when a person's CD4 count is below 200 cells/uL and in those who have or have previously had PCP.[159] People with substantial immunosuppression are also advised to receive prophylactic therapy for toxoplasmosis and Cryptococcus meningitis.[160] Appropriate preventive measures have reduced the rate of these infections by 50% between 1992 and 1997.[161]
Diet
The World Health Organization (WHO) has issued recommendations regarding nutrient requirements in HIV/AIDS.[162] A generally healthy diet is promoted. Some evidence has shown a benefit from micronutrient supplements.[163] Evidence for supplementation with selenium is mixed with some tentative evidence of benefit.[164] There is some evidence that vitamin A supplementation in children reduces mortality and improves growth.[163] In Africa in nutritionally compromised pregnant and lactating women a multivitamin supplementation has improved outcomes for both mothers and children.[163] Dietary intake of micronutrients at RDA levels by HIV-infected adults is recommended by the WHO; higher intake of vitamin A, zinc, and iron can produce adverse effects in HIV positive adults, and is not recommended unless there is documented deficiency.[162][165][166][167]
Alternative medicine
In the US, approximately 60% of people with HIV use various forms of complementary or alternative medicine,[168] even though the effectiveness of most of these therapies has not been established.[169] There is not enough evidence to support the use of herbal medicines.[170] There is insufficient evidence to recommend or support the use of medical cannabis to try to increase appetite or weight gain.[171]
Prognosis


no data
≤ 10
10–25
25–50
50–100
100–500
500–1000
|
1000–2500
2500–5000
5000–7500
7500-10000
10000-50000
≥ 50000
|
HIV/AIDS has become a chronic rather than an acutely fatal disease in many areas of the world.[172] Prognosis varies between people, and both the CD4 count and viral load are useful for predicted outcomes.[25] Without treatment, average survival time after infection with HIV is estimated to be 9 to 11 years, depending on the HIV subtype.[14] After the diagnosis of AIDS, if treatment is not available, survival ranges between 6 and 19 months.[173][174] HAART and appropriate prevention of opportunistic infections reduces the death rate by 80%, and raises the life expectancy for a newly diagnosed young adult to 20–50 years.[172][175][176] This is between two thirds[175] and nearly that of the general population.[26][177] If treatment is started late in the infection, prognosis is not as good:[26] for example, if treatment is begun following the diagnosis of AIDS, life expectancy is ~10–40 years.[26][172] Half of infants born with HIV die before two years of age without treatment.[158]
The primary causes of death from HIV/AIDS are opportunistic infections and cancer, both of which are frequently the result of the progressive failure of the immune system.[161][178] Risk of cancer appears to increase once the CD4 count is below 500/μL.[26] The rate of clinical disease progression varies widely between individuals and has been shown to be affected by a number of factors such as a person's susceptibility and immune function;[179] their access to health care, the presence of co-infections;[173][180] and the particular strain (or strains) of the virus involved.[181][182]
Tuberculosis co-infection is one of the leading causes of sickness and death in those with HIV/AIDS being present in a third of all HIV-infected people and causing 25% of HIV-related deaths.[183] HIV is also one of the most important risk factors for tuberculosis.[184] Hepatitis C is another very common co-infection where each disease increases the progression of the other.[185] The two most common cancers associated with HIV/AIDS are Kaposi's sarcoma and AIDS-related non-Hodgkin's lymphoma.[178]
Even with anti-retroviral treatment, over the long term HIV-infected people may experience neurocognitive disorders,[186] osteoporosis,[187] neuropathy,[188] cancers,[189][190] nephropathy,[191] and cardiovascular disease.[152] Some conditions like lipodystrophy may be caused both by HIV and its treatment.[152]
Epidemiology
No data
<0.10
0.10–0.5
0.5–1
|
1–5
5–15
15–50
|
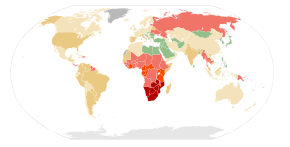
HIV/AIDS is a global pandemic.[193] As of 2014, approximately 37 million people have HIV worldwide with the number of new infections that year being about 2 million.[194] This is down from 3.1 million new infections in 2001.[195] Of these 37 million more than half are women and 2.6 million are less than 15 years old.[194][196] It resulted in about 1.2 million deaths in 2014,[194] down from a peak of 2.2 million in 2005.[140][195]
Sub-Saharan Africa is the region most affected. In 2010, an estimated 68% (22.9 million) of all HIV cases and 66% of all deaths (1.2 million) occurred in this region.[197] This means that about 5% of the adult population is infected[198] and it is believed to be the cause of 10% of all deaths in children.[199] Here in contrast to other regions women compose nearly 60% of cases.[197] South Africa has the largest population of people with HIV of any country in the world at 5.9 million.[197] Life expectancy has fallen in the worst-affected countries due to HIV/AIDS; for example, in 2006 it was estimated that it had dropped from 65 to 35 years in Botswana.[16] Mother-to-child transmission, as of 2013, in Botswana and South Africa has decreased to less than 5% with improvement in many other African nations due to improved access to antiretroviral therapy.[200]
South & South East Asia is the second most affected; in 2010 this region contained an estimated 4 million cases or 12% of all people living with HIV resulting in approximately 250,000 deaths.[198] Approximately 2.4 million of these cases are in India.[197]
In 2008 in the United States approximately 1.2 million people were living with HIV, resulting in about 17,500 deaths. The US Centers for Disease Control and Prevention estimated that in 2008 20% of infected Americans were unaware of their infection.[201] In the United Kingdom as of 2009 there were approximately 86,500 cases which resulted in 516 deaths.[202] In Canada as of 2008 there were about 65,000 cases causing 53 deaths.[203] Between the first recognition of AIDS in 1981 and 2009 it has led to nearly 30 million deaths.[204] Prevalence is lowest in Middle East and North Africa at 0.1% or less, East Asia at 0.1% and Western and Central Europe at 0.2%.[198] The worst affected European countries, in 2009 and 2012 estimates, are Russia, Ukraine, Latvia, Moldova, Portugal and Belarus, in decreasing order of prevalence.[205]
History
Discovery

AIDS was first clinically observed in 1981 in the United States.[34] The initial cases were a cluster of injecting drug users and homosexual men with no known cause of impaired immunity who showed symptoms of Pneumocystis carinii pneumonia (PCP), a rare opportunistic infection that was known to occur in people with very compromised immune systems.[206] Soon thereafter, an unexpected number of homosexual men developed a previously rare skin cancer called Kaposi's sarcoma (KS).[207][208] Many more cases of PCP and KS emerged, alerting U.S. Centers for Disease Control and Prevention (CDC) and a CDC task force was formed to monitor the outbreak.[209]
In the early days, the CDC did not have an official name for the disease, often referring to it by way of the diseases that were associated with it, for example, lymphadenopathy, the disease after which the discoverers of HIV originally named the virus.[210][211] They also used Kaposi's sarcoma and opportunistic infections, the name by which a task force had been set up in 1981.[212] At one point, the CDC coined the phrase "the 4H disease", since the syndrome seemed to affect heroin users, homosexuals, hemophiliacs, and Haitians.[213][214] In the general press, the term "GRID", which stood for gay-related immune deficiency, had been coined.[215] However, after determining that AIDS was not isolated to the gay community,[212] it was realized that the term GRID was misleading and the term AIDS was introduced at a meeting in July 1982.[216] By September 1982 the CDC started referring to the disease as AIDS.[217]
In 1983, two separate research groups led by Robert Gallo and Luc Montagnier declared that a novel retrovirus may have been infecting people with AIDS, and published their findings in the same issue of the journal Science.[218][219] Gallo claimed that a virus his group had isolated from a person with AIDS was strikingly similar in shape to other human T-lymphotropic viruses (HTLVs) his group had been the first to isolate. Gallo's group called their newly isolated virus HTLV-III. At the same time, Montagnier's group isolated a virus from a person presenting with swelling of the lymph nodes of the neck and physical weakness, two characteristic symptoms of AIDS. Contradicting the report from Gallo's group, Montagnier and his colleagues showed that core proteins of this virus were immunologically different from those of HTLV-I. Montagnier's group named their isolated virus lymphadenopathy-associated virus (LAV).[209] As these two viruses turned out to be the same, in 1986, LAV and HTLV-III were renamed HIV.[220]
Origins

Both HIV-1 and HIV-2 are believed to have originated in non-human primates in West-central Africa and were transferred to humans in the early 20th century.[17] HIV-1 appears to have originated in southern Cameroon through the evolution of SIV(cpz), a simian immunodeficiency virus (SIV) that infects wild chimpanzees (HIV-1 descends from the SIVcpz endemic in the chimpanzee subspecies Pan troglodytes troglodytes).[221][222] The closest relative of HIV-2 is SIV(smm), a virus of the sooty mangabey (Cercocebus atys atys), an Old World monkey living in coastal West Africa (from southern Senegal to western Côte d'Ivoire).[87] New World monkeys such as the owl monkey are resistant to HIV-1 infection, possibly because of a genomic fusion of two viral resistance genes.[223] HIV-1 is thought to have jumped the species barrier on at least three separate occasions, giving rise to the three groups of the virus, M, N, and O.[224]
There is evidence that humans who participate in bushmeat activities, either as hunters or as bushmeat vendors, commonly acquire SIV.[225] However, SIV is a weak virus which is typically suppressed by the human immune system within weeks of infection. It is thought that several transmissions of the virus from individual to individual in quick succession are necessary to allow it enough time to mutate into HIV.[226] Furthermore, due to its relatively low person-to-person transmission rate, SIV can only spread throughout the population in the presence of one or more high-risk transmission channels, which are thought to have been absent in Africa before the 20th century.
Specific proposed high-risk transmission channels, allowing the virus to adapt to humans and spread throughout the society, depend on the proposed timing of the animal-to-human crossing. Genetic studies of the virus suggest that the most recent common ancestor of the HIV-1 M group dates back to circa 1910.[227] Proponents of this dating link the HIV epidemic with the emergence of colonialism and growth of large colonial African cities, leading to social changes, including a higher degree of sexual promiscuity, the spread of prostitution, and the accompanying high frequency of genital ulcer diseases (such as syphilis) in nascent colonial cities.[228] While transmission rates of HIV during vaginal intercourse are low under regular circumstances, they are increased many fold if one of the partners suffers from a sexually transmitted infection causing genital ulcers. Early 1900s colonial cities were notable due to their high prevalence of prostitution and genital ulcers, to the degree that, as of 1928, as many as 45% of female residents of eastern Kinshasa were thought to have been prostitutes, and, as of 1933, around 15% of all residents of the same city had syphilis.[228]
An alternative view holds that unsafe medical practices in Africa after World War II, such as unsterile reuse of single use syringes during mass vaccination, antibiotic and anti-malaria treatment campaigns, were the initial vector that allowed the virus to adapt to humans and spread.[226][229][230]
The earliest well-documented case of HIV in a human dates back to 1959 in the Congo.[231] The earliest retrospectively described case of AIDS is believed to have been in Norway beginning in 1966.[232] In July 1960, in the wake its independence, the United Nations recruited Francophone experts and technicians from all over the world to assist in filling administrative gaps left by Belgium, who did not leave behind an African elite to run the country. By 1962, Haitians made up the second largest group of well-educated experts (out of the 48 national groups recruited), that totaled around 4500 in the country.[233][234] Dr. Jacques Pépin, a Quebecer author of The Origins of AIDS, stipulates that Haiti was one of HIV's entry points to the United States and that one of them may have carried HIV back across the Atlantic in the 1960s.[234] Although, the virus may have been present in the United States as early as 1966,[235] the vast majority of infections occurring outside sub-Saharan Africa (including the U.S.) can be traced back to a single unknown individual who became infected with HIV in Haiti and then brought the infection to the United States some time around 1969.[236] The epidemic then rapidly spread among high-risk groups (initially, sexually promiscuous men who have sex with men). By 1978, the prevalence of HIV-1 among homosexual male residents of New York City and San Francisco was estimated at 5%, suggesting that several thousand individuals in the country had been infected.[236]
Society and culture
Stigma

AIDS stigma exists around the world in a variety of ways, including ostracism, rejection, discrimination and avoidance of HIV infected people; compulsory HIV testing without prior consent or protection of confidentiality; violence against HIV infected individuals or people who are perceived to be infected with HIV; and the quarantine of HIV infected individuals.[19] Stigma-related violence or the fear of violence prevents many people from seeking HIV testing, returning for their results, or securing treatment, possibly turning what could be a manageable chronic illness into a death sentence and perpetuating the spread of HIV.[238]
AIDS stigma has been further divided into the following three categories:
- Instrumental AIDS stigma—a reflection of the fear and apprehension that are likely to be associated with any deadly and transmissible illness.[239]
- Symbolic AIDS stigma—the use of HIV/AIDS to express attitudes toward the social groups or lifestyles perceived to be associated with the disease.[239]
- Courtesy AIDS stigma—stigmatization of people connected to the issue of HIV/AIDS or HIV-positive people.[240]
Often, AIDS stigma is expressed in conjunction with one or more other stigmas, particularly those associated with homosexuality, bisexuality, promiscuity, prostitution, and intravenous drug use.[241]
In many developed countries, there is an association between AIDS and homosexuality or bisexuality, and this association is correlated with higher levels of sexual prejudice, such as anti-homosexual/bisexual attitudes.[242] There is also a perceived association between AIDS and all male-male sexual behavior, including sex between uninfected men.[239] However, the dominant mode of spread worldwide for HIV remains heterosexual transmission.[243]
In 2003, as part of an overall reform of marriage and population legislation, it became legal for people with AIDS to marry in China.[244]
Economic impact

HIV/AIDS affects the economics of both individuals and countries.[199] The gross domestic product of the most affected countries has decreased due to the lack of human capital.[199][245] Without proper nutrition, health care and medicine, large numbers of people die from AIDS-related complications. They will not only be unable to work, but will also require significant medical care. It is estimated that as of 2007 there were 12 million AIDS orphans.[199] Many are cared for by elderly grandparents.[246]
Returning to work after beginning treatment for HIV/AIDS is difficult, and affected people often work less than the average worker. Unemployment in people with HIV/AIDS also is associated with suicidal ideation, memory problems, and social isolation; employment increases self-esteem, sense of dignity, confidence, and quality of life. A 2015 Cochrane review found low-quality evidence that antiretroviral treatment helps people with HIV/AIDS work more, and increases the chance that a person with HIV/AIDS will be employed.[247]
By affecting mainly young adults, AIDS reduces the taxable population, in turn reducing the resources available for public expenditures such as education and health services not related to AIDS resulting in increasing pressure for the state's finances and slower growth of the economy. This causes a slower growth of the tax base, an effect that is reinforced if there are growing expenditures on treating the sick, training (to replace sick workers), sick pay and caring for AIDS orphans. This is especially true if the sharp increase in adult mortality shifts the responsibility and blame from the family to the government in caring for these orphans.[246]
At the household level, AIDS causes both loss of income and increased spending on healthcare. A study in Côte d'Ivoire showed that households having a person with HIV/AIDS spent twice as much on medical expenses as other households. This additional expenditure also leaves less income to spend on education and other personal or family investment.[248]
Religion and AIDS
The topic of religion and AIDS has become highly controversial in the past twenty years, primarily because some religious authorities have publicly declared their opposition to the use of condoms.[249][250] The religious approach to prevent the spread of AIDS according to a report by American health expert Matthew Hanley titled The Catholic Church and the Global AIDS Crisis argues that cultural changes are needed including a re-emphasis on fidelity within marriage and sexual abstinence outside of it.[250]
Some religious organizations have claimed that prayer can cure HIV/AIDS. In 2011, the BBC reported that some churches in London were claiming that prayer would cure AIDS, and the Hackney-based Centre for the Study of Sexual Health and HIV reported that several people stopped taking their medication, sometimes on the direct advice of their pastor, leading to a number of deaths.[251] The Synagogue Church Of All Nations advertise an "anointing water" to promote God's healing, although the group deny advising people to stop taking medication.[251]
Media portrayal
One of the first high-profile cases of AIDS was the American Rock Hudson, a gay actor who had been married and divorced earlier in life, who died on October 2, 1985 having announced that he was suffering from the virus on July 25 that year. He had been diagnosed during 1984.[252] A notable British casualty of AIDS that year was Nicholas Eden, a gay politician and son of the late prime minister Anthony Eden.[253] On November 24, 1991, the virus claimed the life of British rock star Freddie Mercury, lead singer of the band Queen, who died from an AIDS-related illness having only revealed the diagnosis on the previous day.[254] However, he had been diagnosed as HIV positive in 1987.[255] One of the first high-profile heterosexual cases of the virus was Arthur Ashe, the American tennis player. He was diagnosed as HIV positive on August 31, 1988, having contracted the virus from blood transfusions during heart surgery earlier in the 1980s. Further tests within 24 hours of the initial diagnosis revealed that Ashe had AIDS, but he did not tell the public about his diagnosis until April 1992.[256] He died as a result on February 6, 1993 at age 49.[257]
Therese Frare's photograph of gay activist David Kirby, as he lay dying from AIDS while surrounded by family, was taken in April 1990. LIFE magazine said the photo became the one image "most powerfully identified with the HIV/AIDS epidemic." The photo was displayed in LIFE magazine, was the winner of the World Press Photo, and acquired worldwide notoriety after being used in a United Colors of Benetton advertising campaign in 1992.[258] In 1996, Johnson Aziga, a Ugandan-born Canadian was diagnosed with HIV, but subsequently had unprotected sex with 11 women without disclosing his diagnosis. By 2003 seven had contracted HIV, and two died from complications related to AIDS.[259][260] Aziga was convicted of first-degree murder and is liable to a life sentence.[261]
Criminal transmission
Criminal transmission of HIV is the intentional or reckless infection of a person with the human immunodeficiency virus (HIV). Some countries or jurisdictions, including some areas of the United States, have laws that criminalize HIV transmission or exposure.[262] Others may charge the accused under laws enacted before the HIV pandemic.
Misconceptions
There are many misconceptions about HIV and AIDS. Three of the most common are that AIDS can spread through casual contact, that sexual intercourse with a virgin will cure AIDS,[263][264][265] and that HIV can infect only gay men and drug users. In 2014, some among the British public wrongly thought one could get HIV from kissing (16%), sharing a glass (5%), spitting (16%), a public toilet seat (4%), and coughing or sneezing (5%).[266] Other misconceptions are that any act of anal intercourse between two uninfected gay men can lead to HIV infection, and that open discussion of HIV and homosexuality in schools will lead to increased rates of AIDS.[267][268]
A small group of individuals continue to dispute the connection between HIV and AIDS,[269] the existence of HIV itself, or the validity of HIV testing and treatment methods.[270][271] These claims, known as AIDS denialism, have been examined and rejected by the scientific community.[272] However, they have had a significant political impact, particularly in South Africa, where the government's official embrace of AIDS denialism (1999–2005) was responsible for its ineffective response to that country's AIDS epidemic, and has been blamed for hundreds of thousands of avoidable deaths and HIV infections.[273][274][275]
Several discredited conspiracy theories have held that HIV was created by scientists, either inadvertently or deliberately. Operation INFEKTION was a worldwide Soviet active measures operation to spread the claim that the United States had created HIV/AIDS. Surveys show that a significant number of people believed – and continue to believe – in such claims.[276]
Research
HIV/AIDS research includes all medical research which attempts to prevent, treat, or cure HIV/AIDS along with fundamental research about the nature of HIV as an infectious agent and AIDS as the disease caused by HIV.
Many governments and research institutions participate in HIV/AIDS research. This research includes behavioral health interventions such as sex education, and drug development, such as research into microbicides for sexually transmitted diseases, HIV vaccines, and antiretroviral drugs. Other medical research areas include the topics of pre-exposure prophylaxis, post-exposure prophylaxis, and circumcision and HIV.
References
- 1 2 3 4 "Stages of HIV". U.S. Department of Health & Human Services. Dec 2010. Retrieved June 13, 2012.
- 1 2 3 4 5 6 Mandell, Bennett, and Dolan (2010). Chapter 121.
- ↑ "HIV Classification: CDC and WHO Staging Systems". Guide for HIV/AIDS Clinical Care. AIDS Education and Training Center Program. Retrieved November 21, 2015.
- ↑ "World AIDS Day". World Health Organization. Retrieved June 16, 2015.
- ↑ Sepkowitz KA (June 2001). "AIDS—the first 20 years". N. Engl. J. Med. 344 (23): 1764–72. doi:10.1056/NEJM200106073442306. PMID 11396444.
- ↑ editors, Alexander Krämer, Mirjam Kretzschmar, Klaus Krickeberg, (2010). Modern infectious disease epidemiology concepts, methods, mathematical models, and public health (Online-Ausg. ed.). New York: Springer. p. 88. ISBN 9780387938356.
- ↑ Wilhelm Kirch (2008). Encyclopedia of public health. New York: Springer. pp. 676–677. ISBN 9781402056130.
- 1 2 3 4 5 6 "HIV/AIDS Fact sheet N°360". WHO. November 2015. Retrieved 11 February 2016.
- 1 2 3 4 "About HIV/AIDS". CDC. December 6, 2015. Retrieved 11 February 2016.
- 1 2 3 4 5 6 7 8 9 Markowitz, edited by William N. Rom ; associate editor, Steven B. (2007). Environmental and occupational medicine (4th ed.). Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins. p. 745. ISBN 978-0-7817-6299-1.
- ↑ "HIV and Its Transmission". Centers for Disease Control and Prevention. 2003. Archived from the original on February 4, 2005. Retrieved May 23, 2006.
- 1 2 UNAIDS (May 18, 2012). "The quest for an HIV vaccine".
- 1 2 Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. (PDF). WHO. 2015. p. 13. ISBN 9789241509565.
- 1 2 UNAIDS, WHO (December 2007). "2007 AIDS epidemic update" (PDF). Retrieved March 12, 2008.
- ↑ "Basic Statistics". CDC. November 3, 2015. Retrieved 11 February 2016.
- 1 2 Kallings LO (2008). "The first postmodern pandemic: 25 years of HIV/AIDS". Journal of Internal Medicine. 263 (3): 218–43. doi:10.1111/j.1365-2796.2007.01910.x. PMID 18205765.(subscription required)
- 1 2 Sharp, PM; Hahn, BH (September 2011). "Origins of HIV and the AIDS Pandemic". Cold Spring Harbor perspectives in medicine. 1 (1): a006841. doi:10.1101/cshperspect.a006841. PMC 3234451
 . PMID 22229120.
. PMID 22229120. - ↑ Gallo RC (2006). "A reflection on HIV/AIDS research after 25 years". Retrovirology. 3 (1): 72. doi:10.1186/1742-4690-3-72. PMC 1629027
 . PMID 17054781.
. PMID 17054781. - 1 2 3 "The impact of AIDS on people and societies" (PDF). 2006 Report on the global AIDS epidemic. UNAIDS. 2006. ISBN 92-9173-479-9. Retrieved June 14, 2006.
- ↑ "Myth Busters". Retrieved 14 February 2016.
- ↑ McCullom, Rob (26 Feb 2013). "An African Pope Won't Change the Vatican's Views on Condoms and AIDS previousnext An African Pope Won't Change the Vatican's Views on Condoms and AIDS". The Atlantic. Retrieved 14 February 2016.
- ↑ Harden, Victoria Angela (2012). AIDS at 30: A History. Potomac Books Inc. p. 324. ISBN 1-59797-294-0.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. (PDF). Geneva: World Health Organization. 2007. pp. 6–16. ISBN 978-92-4-159562-9.
- ↑ Diseases and disorders. Tarrytown, NY: Marshall Cavendish. 2008. p. 25. ISBN 978-0-7614-7771-6.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Mandell, Bennett, and Dolan (2010). Chapter 118.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Vogel, M; Schwarze-Zander, C; Wasmuth, JC; Spengler, U; Sauerbruch, T; Rockstroh, JK (July 2010). "The treatment of patients with HIV". Deutsches Ärzteblatt international. 107 (28–29): 507–15; quiz 516. doi:10.3238/arztebl.2010.0507. PMC 2915483
 . PMID 20703338.
. PMID 20703338. - ↑ Evian, Clive (2006). Primary HIV/AIDS care: a practical guide for primary health care personnel in a clinical and supportive setting (Updated 4th ed.). Houghton [South Africa]: Jacana. p. 29. ISBN 978-1-77009-198-6.
- ↑ Charles B. Hicks, MD (2001). Jacques W. A. J. Reeders & Philip Charles Goodman, ed. Radiology of AIDS. Berlin [u.a.]: Springer. p. 19. ISBN 978-3-540-66510-6.
- ↑ Elliott, Tom (2012). Lecture Notes: Medical Microbiology and Infection. John Wiley & Sons. p. 273. ISBN 978-1-118-37226-5.
- 1 2 Blankson, JN (March 2010). "Control of HIV-1 replication in elite suppressors". Discovery medicine. 9 (46): 261–6. PMID 20350494.
- ↑ Walker, BD (Aug–Sep 2007). "Elite control of HIV Infection: implications for vaccines and treatment". Topics in HIV medicine : a publication of the International AIDS Society, USA. 15 (4): 134–6. PMID 17720999.
- ↑ Holmes CB, Losina E, Walensky RP, Yazdanpanah Y, Freedberg KA (2003). "Review of human immunodeficiency virus type 1-related opportunistic infections in sub-Saharan Africa". Clin. Infect. Dis. 36 (5): 656–662. doi:10.1086/367655. PMID 12594648.
- ↑ Chu, C; Selwyn, PA (February 15, 2011). "Complications of HIV infection: a systems-based approach". American family physician. 83 (4): 395–406. PMID 21322514.
- 1 2 3 4 5 Mandell, Bennett, and Dolan (2010). Chapter 169.
- ↑ Mittal, R; Rath, S; Vemuganti, GK (Jul 2013). "Ocular surface squamous neoplasia – Review of etio-pathogenesis and an update on clinico-pathological diagnosis.". Saudi Journal of Ophthalmology. 27 (3): 177–86. doi:10.1016/j.sjopt.2013.07.002. PMID 24227983.
- ↑ "AIDS". MedlinePlus. A.D.A.M. Retrieved June 14, 2012.
- ↑ Sestak K (July 2005). "Chronic diarrhea and AIDS: insights into studies with non-human primates". Curr. HIV Res. 3 (3): 199–205. doi:10.2174/1570162054368084. PMID 16022653.
- ↑ Murray ED, Buttner N, Price BH (2012). "Depression and Psychosis in Neurological Practice". In Bradley WG, Daroff RB, Fenichel GM, Jankovic J. Bradley's Neurology in Clinical Practice: Expert Consult – Online and Print, 6e (Bradley, Neurology in Clinical Practice e-dition 2v Set). 1 (6th ed.). Philadelphia, PA: Elsevier/Saunders. p. 101. ISBN 1-4377-0434-4.
- 1 2 Smith DK, Grohskopf LA, Black RJ, Auerbach JD, Veronese F, Struble KA, Cheever L, Johnson M, Paxton LA, Onorato IM, Greenberg AE (21 January 2005). "Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the U.S. Department of Health and Human Services.". MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 54 (RR-2): 1–20. PMID 15660015.
- ↑ Coovadia H (2004). "Antiretroviral agents—how best to protect infants from HIV and save their mothers from AIDS". N. Engl. J. Med. 351 (3): 289–292. doi:10.1056/NEJMe048128. PMID 15247337.
- ↑ Kripke C (1 August 2007). "Antiretroviral prophylaxis for occupational exposure to HIV.". American family physician. 76 (3): 375–6. PMID 17708137.
- 1 2 3 4 Dosekun O, Fox J (July 2010). "An overview of the relative risks of different sexual behaviours on HIV transmission.". Current opinion in HIV and AIDS. 5 (4): 291–7. doi:10.1097/COH.0b013e32833a88a3. PMID 20543603.
- ↑ Cunha, Burke (2012). Antibiotic Essentials 2012 (11 ed.). Jones & Bartlett Publishers. p. 303. ISBN 9781449693831.
- 1 2 Boily MC, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, Alary M (February 2009). "Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies.". The Lancet infectious diseases. 9 (2): 118–29. doi:10.1016/S1473-3099(09)70021-0. PMID 19179227.
- ↑ Baggaley RF, White RG, Boily MC (December 2008). "Systematic review of orogenital HIV-1 transmission probabilities.". International Journal of Epidemiology. 37 (6): 1255–65. doi:10.1093/ije/dyn151. PMC 2638872
 . PMID 18664564.
. PMID 18664564. - 1 2 3 Kripke, C (August 1, 2007). "Antiretroviral prophylaxis for occupational exposure to HIV". American family physician. 76 (3): 375–6. PMID 17708137.
- ↑ van der Kuyl, AC; Cornelissen, M (September 24, 2007). "Identifying HIV-1 dual infections". Retrovirology. 4: 67. doi:10.1186/1742-4690-4-67. PMC 2045676
 . PMID 17892568.
. PMID 17892568. - ↑ "Gay and Bisexual Men HIV by Group". www.cdc.gov. Retrieved 15 May 2016.
- 1 2 3 4 5 6 7 Boily MC, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, Alary M (February 2009). "Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies". The Lancet Infectious Diseases. 9 (2): 118–129. doi:10.1016/S1473-3099(09)70021-0. PMID 19179227.
- ↑ Beyrer, C; Baral, SD; van Griensven, F; Goodreau, SM; Chariyalertsak, S; Wirtz, AL; Brookmeyer, R (Jul 28, 2012). "Global epidemiology of HIV infection in men who have sex with men". Lancet. 380 (9839): 367–77. doi:10.1016/S0140-6736(12)60821-6. PMID 22819660.
- ↑ Yu, M; Vajdy, M (August 2010). "Mucosal HIV transmission and vaccination strategies through oral compared with vaginal and rectal routes". Expert opinion on biological therapy. 10 (8): 1181–95. doi:10.1517/14712598.2010.496776. PMC 2904634
 . PMID 20624114.
. PMID 20624114. - ↑ Stürchler, Dieter A. (2006). Exposure a guide to sources of infections. Washington, DC: ASM Press. p. 544. ISBN 978-1-55581-376-5.
- ↑ al.], edited by Richard Pattman (2010). Oxford handbook of genitourinary medicine, HIV, and sexual health (2nd ed.). Oxford: Oxford University Press. p. 95. ISBN 978-0-19-957166-6.
- 1 2 3 Dosekun, O; Fox, J (July 2010). "An overview of the relative risks of different sexual behaviours on HIV transmission". Current Opinion in HIV and AIDS. 5 (4): 291–7. doi:10.1097/COH.0b013e32833a88a3. PMID 20543603.
- 1 2 Ng, BE; Butler, LM; Horvath, T; Rutherford, GW (March 16, 2011). Butler, Lisa M, ed. "Population-based biomedical sexually transmitted infection control interventions for reducing HIV infection". Cochrane database of systematic reviews (Online) (3): CD001220. doi:10.1002/14651858.CD001220.pub3. PMID 21412869.
- ↑ Anderson, J (February 2012). "Women and HIV: motherhood and more". Current Opinion in Infectious Diseases. 25 (1): 58–65. doi:10.1097/QCO.0b013e32834ef514. PMID 22156896.
- ↑ Kerrigan, Deanna (2012). The Global HIV Epidemics among Sex Workers. World Bank Publications. p. 1. ISBN 978-0-8213-9775-6.
- ↑ Aral, Sevgi (2013). The New Public Health and STD/HIV Prevention: Personal, Public and Health Systems Approaches. Springer. p. 120. ISBN 978-1-4614-4526-5.
- ↑ Klimas, N; Koneru, AO; Fletcher, MA (June 2008). "Overview of HIV". Psychosomatic Medicine. 70 (5): 523–30. doi:10.1097/PSY.0b013e31817ae69f. PMID 18541903.
- ↑ Draughon, JE; Sheridan, DJ (2012). "Nonoccupational post exposure prophylaxis following sexual assault in industrialized low-HIV-prevalence countries: a review". Psychology, health & medicine. 17 (2): 235–54. doi:10.1080/13548506.2011.579984. PMID 22372741.
- 1 2 Baggaley, RF; Boily, MC; White, RG; Alary, M (April 4, 2006). "Risk of HIV-1 transmission for parenteral exposure and blood transfusion: a systematic review and meta-analysis". AIDS (London, England). 20 (6): 805–12. doi:10.1097/01.aids.0000218543.46963.6d. PMID 16549963.
- ↑ "HIV in the United States: An Overview". Center for Disease Control and Prevention. March 2012.
- ↑ "Will I need a blood transfusion?" (PDF). NHS patient information. National Health Services. 2011. Retrieved August 29, 2012.
- ↑ Centers for Disease Control and Prevention, (CDC) (October 22, 2010). "HIV transmission through transfusion --- Missouri and Colorado, 2008.". MMWR. Morbidity and mortality weekly report. 59 (41): 1335–9. PMID 20966896.
- ↑ UNAIDS 2011 pg. 60–70
- ↑ "Blood safety ... for too few". WHO. 2001. Archived from the original on January 17, 2005.
- ↑ Simonds, RJ (November 1993). "HIV transmission by organ and tissue transplantation". AIDS. 7 Suppl 2: S35–8. doi:10.1097/00002030-199311002-00008. PMID 8161444.
- 1 2 3 Reid, SR (August 28, 2009). "Injection drug use, unsafe medical injections, and HIV in Africa: a systematic review". Harm reduction journal. 6: 24. doi:10.1186/1477-7517-6-24. PMC 2741434
 . PMID 19715601.
. PMID 19715601. - 1 2 "Basic Information about HIV and AIDS". Center for Disease Control and Prevention. April 2012.
- ↑ Crans, Wayne J. (June 1, 2010). "Why Mosquitoes Cannot Transmit AIDS". rci.rutgers.edu. Rutgers University. New Jersey Agricultural Experiment Station Publication No. H-40101-01-93. Archived from the original on March 29, 2014. Retrieved March 29, 2014.
- 1 2 3 4 5 6 Coutsoudis, A; Kwaan, L; Thomson, M (October 2010). "Prevention of vertical transmission of HIV-1 in resource-limited settings". Expert review of anti-infective therapy. 8 (10): 1163–75. doi:10.1586/eri.10.94. PMID 20954881.
- ↑ "Fluids of transmission". AIDS.gov. United States Department of Health and Human Services. November 1, 2011. Retrieved September 14, 2012.
- 1 2 Thorne, C; Newell, ML (June 2007). "HIV". Seminars in fetal & neonatal medicine. 12 (3): 174–81. doi:10.1016/j.siny.2007.01.009. PMID 17321814.
- ↑ White, AB; Mirjahangir, JF; Horvath, H; Anglemyer, A; Read, JS (Oct 4, 2014). "Antiretroviral interventions for preventing breast milk transmission of HIV.". The Cochrane database of systematic reviews. 10: CD011323. doi:10.1002/14651858.CD011323. PMID 25280769.
- ↑ Alimonti JB, Ball TB, Fowke KR (2003). "Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS". J. Gen. Virol. 84 (7): 1649–1661. doi:10.1099/vir.0.19110-0. PMID 12810858.
- ↑ International Committee on Taxonomy of Viruses (2002). "61.0.6. Lentivirus". National Institutes of Health. Archived from the original on April 18, 2006. Retrieved June 25, 2012.
- ↑ International Committee on Taxonomy of Viruses (2002). "61. Retroviridae". National Institutes of Health. Archived from the original on December 17, 2001. Retrieved June 25, 2012.
- ↑ Lévy, J. A. (1993). "HIV pathogenesis and long-term survival". AIDS. 7 (11): 1401–10. doi:10.1097/00002030-199311000-00001. PMID 8280406.
- ↑ Smith, Johanna A.; Daniel, René (Division of Infectious Diseases, Center for Human Virology, Thomas Jefferson University, Philadelphia) (2006). "Following the path of the virus: the exploitation of host DNA repair mechanisms by retroviruses". ACS Chem Biol. 1 (4): 217–26. doi:10.1021/cb600131q. PMID 17163676.
- ↑ Martínez, edited by Miguel Angel (2010). RNA interference and viruses : current innovations and future trends. Norfolk: Caister Academic Press. p. 73. ISBN 978-1-904455-56-1.
- ↑ Gerald B. Pier, ed. (2004). Immunology, infection, and immunity. Washington, D.C.: ASM Press. p. 550. ISBN 978-1-55581-246-1.
- 1 2 3 Zhang C, Zhou S, Groppelli E, Pellegrino P, Williams I, Borrow P, Chain BM, Jolly C (2015). "Hybrid Spreading Mechanisms and T Cell Activation Shape the Dynamics of HIV-1 Infection". PLOS Computational Biology. 11 (4): e1004179. doi:10.1371/journal.pcbi.1004179. PMC 4383537
 . PMID 25837979.
. PMID 25837979. - ↑ Jolly C, Kashefi K, Hollinshead M, Sattentau QJ (2004). "HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse". Journal of Experimental Medicine. 199 (2): 283–293. doi:10.1084/jem.20030648. PMC 2211771
 . PMID 14734528.
. PMID 14734528. - ↑ Sattentau Q (2008). "Avoiding the void: cell-to-cell spread of human viruses". Nature Reviews Microbiology. 6 (11): 815–826. doi:10.1038/nrmicro1972. PMID 18923409.
- ↑ Sigal A, Kim JT, Balazs AB, Dekel E, Mayo A, Milo R, Baltimore D (2011). "Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy". Nature. 477 (7362): 95–98. doi:10.1038/nature10347. PMID 21849975.
- ↑ Gilbert, PB; et al. (February 28, 2003). "Comparison of HIV-1 and HIV-2 infectivity from a prospective cohort study in Senegal". Statistics in Medicine. 22 (4): 573–593. doi:10.1002/sim.1342. PMID 12590415.
- 1 2 Reeves, J. D.; Doms, R. W (2002). "Human Immunodeficiency Virus Type 2". J. Gen. Virol. 83 (Pt 6): 1253–65. doi:10.1099/0022-1317-83-6-1253. PMID 12029140.
- ↑ Piatak, M., Jr, Saag, M. S., Yang, L. C., Clark, S. J., Kappes, J. C., Luk, K. C., Hahn, B. H., Shaw, G. M. and Lifson, J.D. (1993). "High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR". Science. 259 (5102): 1749–1754. Bibcode:1993Sci...259.1749P. doi:10.1126/science.8096089. PMID 8096089.
- ↑ Pantaleo G, Demarest JF, Schacker T, Vaccarezza M, Cohen OJ, Daucher M, Graziosi C, Schnittman SS, Quinn TC, Shaw GM, Perrin L, Tambussi G, Lazzarin A, Sekaly RP, Soudeyns H, Corey L, Fauci AS (1997). "The qualitative nature of the primary immune response to HIV infection is a prognosticator of disease progression independent of the initial level of plasma viremia". Proc Natl Acad Sci U S A. 94 (1): 254–258. Bibcode:1997PNAS...94..254P. doi:10.1073/pnas.94.1.254. PMC 19306
 . PMID 8990195.
. PMID 8990195. - ↑ Hel Z, McGhee JR, Mestecky J (June 2006). "HIV infection: first battle decides the war". Trends Immunol. 27 (6): 274–81. doi:10.1016/j.it.2006.04.007. PMID 16679064.
- ↑ Pillay, Deenan; Genetti, Anna Maria; Weiss, Robin A. (2007). "Human Immunodeficiency Viruses". In Zuckerman, Arie J.; et al. Principles and practice of clinical virology (6th ed.). Hoboken, N.J.: Wiley. p. 905. ISBN 978-0-470-51799-4.
- ↑ Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M (September 2004). "Primary HIV-1 infection is associated with preferential depletion of CD4+ T cells from effector sites in the gastrointestinal tract". J. Exp. Med. 200 (6): 761–70. doi:10.1084/jem.20041196. PMC 2211967
 . PMID 15365095.
. PMID 15365095. - ↑ Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC (September 2004). "CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract". J. Exp. Med. 200 (6): 749–59. doi:10.1084/jem.20040874. PMC 2211962
 . PMID 15365096.
. PMID 15365096. - ↑ Olson, WC; Jacobson, JM (March 2009). "CCR5 monoclonal antibodies for HIV-1 therapy.". Current opinion in HIV and AIDS. 4 (2): 104–11. doi:10.1097/COH.0b013e3283224015. PMID 19339948.
- 1 2 editor, Julio Aliberti, (2011). Control of Innate and Adaptive Immune Responses During Infectious Diseases. New York, NY: Springer Verlag. p. 145. ISBN 978-1-4614-0483-5.
- ↑ Appay V, Sauce D (January 2008). "Immune activation and inflammation in HIV-1 infection: causes and consequences". J. Pathol. 214 (2): 231–41. doi:10.1002/path.2276. PMID 18161758.
- ↑ Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC (December 2006). "Microbial translocation is a cause of systemic immune activation in chronic HIV infection". Nat. Med. 12 (12): 1365–71. doi:10.1038/nm1511. PMID 17115046.
- ↑ Moyer,, Virginia A. (April 2013). "Screening for HIV: U.S. Preventive Services Task Force Recommendation Statement". Annals of Internal Medicine. doi:10.7326/0003-4819-159-1-201307020-00645.
- 1 2 Kellerman, S; Essajee, S (Jul 20, 2010). "HIV testing for children in resource-limited settings: what are we waiting for?". PLOS Medicine. 7 (7): e1000285. doi:10.1371/journal.pmed.1000285. PMC 2907270
 . PMID 20652012.
. PMID 20652012. - 1 2 3 UNAIDS 2011 pg. 70–80
- 1 2 3 Schneider, E; Whitmore, S; Glynn, KM; Dominguez, K; Mitsch, A; McKenna, MT; Centers for Disease Control and Prevention, (CDC) (December 5, 2008). "Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years--United States, 2008". MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 57 (RR–10): 1–12. PMID 19052530.
- 1 2 3 Centers for Disease Control and Prevention, (CDC) (April 11, 2014). "Revised surveillance case definition for HIV infection—United States, 2014.". MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 63 (RR-03): 1–10. PMID 24717910.
- ↑ Crosby, R; Bounse, S (March 2012). "Condom effectiveness: where are we now?". Sexual health. 9 (1): 10–7. doi:10.1071/SH11036. PMID 22348628.
- ↑ "Condom Facts and Figures". WHO. August 2003. Retrieved January 17, 2006.
- ↑ Gallo, MF; Kilbourne-Brook, M; Coffey, PS (March 2012). "A review of the effectiveness and acceptability of the female condom for dual protection". Sexual health. 9 (1): 18–26. doi:10.1071/SH11037. PMID 22348629.
- 1 2 Celum, C; Baeten, JM (February 2012). "Tenofovir-based pre-exposure prophylaxis for HIV prevention: evolving evidence". Current Opinion in Infectious Diseases. 25 (1): 51–7. doi:10.1097/QCO.0b013e32834ef5ef. PMC 3266126
 . PMID 22156901.
. PMID 22156901. - ↑ Baptista, M; Ramalho-Santos, J (November 1, 2009). "Spermicides, microbicides and antiviral agents: recent advances in the development of novel multi-functional compounds". Mini reviews in medicinal chemistry. 9 (13): 1556–67. doi:10.2174/138955709790361548. PMID 20205637.
- ↑ Siegfried, N; Muller, M; Deeks, JJ; Volmink, J (April 15, 2009). Siegfried, Nandi, ed. "Male circumcision for prevention of heterosexual acquisition of HIV in men". Cochrane database of systematic reviews (Online) (2): CD003362. doi:10.1002/14651858.CD003362.pub2. PMID 19370585.
- ↑ "WHO and UNAIDS announce recommendations from expert consultation on male circumcision for HIV prevention". World Health Organization. Mar 28, 2007.
- ↑ Larke, N (May 27, 2010). "Male circumcision, HIV and sexually transmitted infections: a review". British journal of nursing (Mark Allen Publishing). 19 (10): 629–34. doi:10.12968/bjon.2010.19.10.48201. PMID 20622758.
- ↑ Eaton, L; Kalichman, SC (November 2009). "Behavioral aspects of male circumcision for the prevention of HIV infection". Current HIV/AIDS reports. 6 (4): 187–93. doi:10.1007/s11904-009-0025-9. PMC 3557929
 . PMID 19849961.(subscription required)
. PMID 19849961.(subscription required) - ↑ Kim, HH; Li, PS; Goldstein, M (November 2010). "Male circumcision: Africa and beyond?". Current Opinion in Urology. 20 (6): 515–9. doi:10.1097/MOU.0b013e32833f1b21. PMID 20844437.
- ↑ Templeton, DJ; Millett, GA; Grulich, AE (February 2010). "Male circumcision to reduce the risk of HIV and sexually transmitted infections among men who have sex with men". Current Opinion in Infectious Diseases. 23 (1): 45–52. doi:10.1097/QCO.0b013e328334e54d. PMID 19935420.
- ↑ Wiysonge, Charles Shey; Kongnyuy, Eugene J; Shey, Muki; Muula, Adamson S; Navti, Osric B; Akl, Elie A; Lo, Ying-Ru (June 15, 2011). Wiysonge, Charles Shey, ed. "Male circumcision for prevention of homosexual acquisition of HIV in men". Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd (6): CD007496. doi:10.1002/14651858.CD007496.pub2. PMID 21678366.
- 1 2 Marrazzo, JM; del Rio, C; Holtgrave, DR; Cohen, MS; Kalichman, SC; Mayer, KH; Montaner, JS; Wheeler, DP; Grant, RM; Grinsztejn, B; Kumarasamy, N; Shoptaw, S; Walensky, RP; Dabis, F; Sugarman, J; Benson, CA; International Antiviral Society-USA, Panel (Jul 23–30, 2014). "HIV prevention in clinical care settings: 2014 recommendations of the International Antiviral Society-USA Panel.". JAMA: The Journal of the American Medical Association. 312 (4): 390–409. doi:10.1001/jama.2014.7999. PMID 25038358.
- ↑ Eaton LA, Kalichman S (December 2007). "Risk compensation in HIV prevention: implications for vaccines, microbicides, and other biomedical HIV prevention technologies". Curr HIV/AIDS Rep. 4 (4): 165–72. doi:10.1007/s11904-007-0024-7. PMC 2937204
 . PMID 18366947.
. PMID 18366947. - ↑ Underhill K, Operario D, Montgomery P (2008). Operario, Don, ed. "Abstinence-only programs for HIV infection prevention in high-income countries". Cochrane Database of Systematic Reviews (4): CD005421. doi:10.1002/14651858.CD005421.pub2. PMID 17943855.
- ↑ Tolli, MV (May 28, 2012). "Effectiveness of peer education interventions for HIV prevention, adolescent pregnancy prevention and sexual health promotion for young people: a systematic review of European studies". Health education research. 27 (5): 904–13. doi:10.1093/her/cys055. PMID 22641791.
- ↑ Ljubojević, S; Lipozenčić, J (2010). "Sexually transmitted infections and adolescence". Acta Dermatovenerologica Croatica. 18 (4): 305–10. PMID 21251451.
- ↑ Patel VL, Yoskowitz NA, Kaufman DR, Shortliffe EH (2008). "Discerning patterns of human immunodeficiency virus risk in healthy young adults". Am J Med. 121 (4): 758–764. doi:10.1016/j.amjmed.2008.04.022. PMC 2597652
 . PMID 18724961.
. PMID 18724961. - ↑ Fonner, VA; Denison, J; Kennedy, CE; O'Reilly, K; Sweat, M (Sep 12, 2012). "Voluntary counseling and testing (VCT) for changing HIV-related risk behavior in developing countries.". The Cochrane database of systematic reviews. 9: CD001224. doi:10.1002/14651858.CD001224.pub4. PMID 22972050.
- 1 2 Anglemyer, A; Rutherford, GW; Horvath, T; Baggaley, RC; Egger, M; Siegfried, N (April 30, 2013). "Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples.". The Cochrane database of systematic reviews. 4: CD009153. doi:10.1002/14651858.CD009153.pub3. PMID 23633367.
- ↑ Chou R, Selph S, Dana T, et al. (November 2012). "Screening for HIV: systematic review to update the 2005 U.S. Preventive Services Task Force recommendation". Annals of Internal Medicine. 157 (10): 706–18. doi:10.7326/0003-4819-157-10-201211200-00007. PMID 23165662.
- ↑ Choopanya, Kachit; Martin, Michael; Suntharasamai, Pravan; Sangkum, Udomsak; Mock, Philip A; Leethochawalit, Manoj; Chiamwongpaet, Sithisat; Kitisin, Praphan; Natrujirote, Pitinan; Kittimunkong, Somyot; Chuachoowong, Rutt; Gvetadze, Roman J; McNicholl, Janet M; Paxton, Lynn A; Curlin, Marcel E; Hendrix, Craig W; Vanichseni, Suphak (June 1, 2013). "Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial". The Lancet. 381 (9883): 2083–2090. doi:10.1016/S0140-6736(13)61127-7. PMID 23769234.
- ↑ Centers for Disease Control (CDC) (August 1987). "Recommendations for prevention of HIV transmission in health-care settings". MMWR. 36 (Suppl 2): 1S–18S. PMID 3112554.
- 1 2 Kurth, AE; Celum, C; Baeten, JM; Vermund, SH; Wasserheit, JN (March 2011). "Combination HIV prevention: significance, challenges, and opportunities". Current HIV/AIDS reports. 8 (1): 62–72. doi:10.1007/s11904-010-0063-3. PMC 3036787
 . PMID 20941553.
. PMID 20941553. - ↑ MacArthur, G. J.; Minozzi, S.; Martin, N.; Vickerman, P.; Deren, S.; Bruneau, J.; Degenhardt, L.; Hickman, M. (October 4, 2012). "Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis". BMJ. 345 (oct03 3): e5945–e5945. doi:10.1136/bmj.e5945.
- 1 2 [No authors listed] (April 2012). "HIV exposure through contact with body fluids". Prescrire Int. 21 (126): 100–1, 103–5. PMID 22515138.
- ↑ Kuhar DT, Henderson DK, Struble KA, et al. (September 2013). "Updated US Public Health Service Guidelines for the Management of Occupational Exposures to Human Immunodeficiency Virus and Recommendations for Postexposure Prophylaxis". Infect Control Hosp Epidemiol. 34 (9): 875–92. doi:10.1086/672271. PMID 23917901.
- ↑ Linden, JA (September 1, 2011). "Clinical practice. Care of the adult patient after sexual assault". The New England Journal of Medicine. 365 (9): 834–41. doi:10.1056/NEJMcp1102869. PMID 21879901.
- ↑ Young, TN; Arens, FJ; Kennedy, GE; Laurie, JW; Rutherford, G (January 24, 2007). Young, Taryn, ed. "Antiretroviral post-exposure prophylaxis (PEP) for occupational HIV exposure". Cochrane database of systematic reviews (Online) (1): CD002835. doi:10.1002/14651858.CD002835.pub3. PMID 17253483.
- ↑ Siegfried, N; van der Merwe, L; Brocklehurst, P; Sint, TT (July 6, 2011). Siegfried, Nandi, ed. "Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection". Cochrane database of systematic reviews (Online) (7): CD003510. doi:10.1002/14651858.CD003510.pub3. PMID 21735394.
- ↑ "WHO HIV and Infant Feeding Technical Consultation Held on behalf of the Inter-agency Task Team (IATT) on Prevention of HIV – Infections in Pregnant Women, Mothers and their Infants – Consensus statement" (PDF). October 25–27, 2006. Archived (PDF) from the original on April 9, 2008. Retrieved March 12, 2008.
- ↑ Horvath, T; Madi, BC; Iuppa, IM; Kennedy, GE; Rutherford, G; Read, JS (January 21, 2009). Horvath, Tara, ed. "Interventions for preventing late postnatal mother-to-child transmission of HIV". Cochrane database of systematic reviews (Online) (1): CD006734. doi:10.1002/14651858.CD006734.pub2. PMID 19160297.
- ↑ "WHO validates elimination of mother-to-child transmission of HIV and syphilis in Cuba". WHO. June 30, 2015. Retrieved August 30, 2015.
- ↑ Reynell, L; Trkola, A (March 2, 2012). "HIV vaccines: an attainable goal?". Swiss Medical Weekly. 142: w13535. doi:10.4414/smw.2012.13535. PMID 22389197.
- ↑ U.S. Army Office of the Surgeon General (March 21, 2011). "HIV Vaccine Trial in Thai Adults". ClinicalTrials.gov. Retrieved June 28, 2011.
- ↑ U.S. Army Office of the Surgeon General (June 2, 2010). "Follow up of Thai Adult Volunteers With Breakthrough HIV Infection After Participation in a Preventive HIV Vaccine Trial". ClinicalTrials.gov.
- ↑ May, MT; Ingle, SM (December 2011). "Life expectancy of HIV-positive adults: a review". Sexual health. 8 (4): 526–33. doi:10.1071/SH11046. PMID 22127039.
- 1 2 3 UNAIDS 2011 pg. 1–10
- 1 2 3 4 Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach (PDF). World Health Organization. 2010. pp. 19–20. ISBN 978-92-4-159976-4.
- 1 2 Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection (PDF). World Health Organization. 2013. pp. 28–30. ISBN 978-92-4-150572-7.
- ↑ "Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents" (pdf). Department of Health and Human Services. February 12, 2013. p. i. Retrieved January 3, 2014.
- ↑ When To Start, Consortium; Sterne, JA; May, M; Costagliola, D; de Wolf, F; Phillips, AN; Harris, R; Funk, MJ; Geskus, RB; Gill, J; Dabis, F; Miró, JM; Justice, AC; Ledergerber, B; Fätkenheuer, G; Hogg, RS; Monforte, AD; Saag, M; Smith, C; Staszewski, S; Egger, M; Cole, SR (April 18, 2009). "Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies". Lancet. 373 (9672): 1352–63. doi:10.1016/S0140-6736(09)60612-7. PMC 2670965
 . PMID 19361855.
. PMID 19361855. - ↑ Beard, J; Feeley, F; Rosen, S (November 2009). "Economic and quality of life outcomes of antiretroviral therapy for HIV/AIDS in developing countries: a systematic literature review". AIDS Care. 21 (11): 1343–56. doi:10.1080/09540120902889926. PMID 20024710.
- ↑ Orrell, C (November 2005). "Antiretroviral adherence in a resource-poor setting". Current HIV/AIDS reports. 2 (4): 171–6. doi:10.1007/s11904-005-0012-8. PMID 16343374.
- ↑ Malta, M; Strathdee, SA; Magnanini, MM; Bastos, FI (August 2008). "Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: a systematic review". Addiction (Abingdon, England). 103 (8): 1242–57. doi:10.1111/j.1360-0443.2008.02269.x. PMID 18855813.
- ↑ Nachega, JB; Marconi, VC; van Zyl, GU; Gardner, EM; Preiser, W; Hong, SY; Mills, EJ; Gross, R (April 2011). "HIV treatment adherence, drug resistance, virologic failure: evolving concepts". Infectious disorders drug targets. 11 (2): 167–74. doi:10.2174/187152611795589663. PMID 21406048.
- ↑ Orsi, F; d'almeida, C (May 2010). "Soaring antiretroviral prices, TRIPS and TRIPS flexibilities: a burning issue for antiretroviral treatment scale-up in developing countries". Current Opinion in HIV and AIDS. 5 (3): 237–41. doi:10.1097/COH.0b013e32833860ba. PMID 20539080.
- ↑ Nachega, JB; Mills, EJ; Schechter, M (January 2010). "Antiretroviral therapy adherence and retention in care in middle-income and low-income countries: current status of knowledge and research priorities". Current Opinion in HIV and AIDS. 5 (1): 70–7. doi:10.1097/COH.0b013e328333ad61. PMID 20046150.
- 1 2 3 Montessori, V., Press, N., Harris, M., Akagi, L., Montaner, J. S. (2004). "Adverse effects of antiretroviral therapy for HIV infection". CMAJ. 170 (2): 229–238. PMC 315530
 . PMID 14734438.
. PMID 14734438. - 1 2 3 Burgoyne RW, Tan DH (March 2008). "Prolongation and quality of life for HIV-infected adults treated with highly active antiretroviral therapy (HAART): a balancing act". J. Antimicrob. Chemother. 61 (3): 469–73. doi:10.1093/jac/dkm499. PMID 18174196.
- ↑ Barbaro, G; Barbarini, G (December 2011). "Human immunodeficiency virus & cardiovascular risk". The Indian journal of medical research. 134 (6): 898–903. doi:10.4103/0971-5916.92634. PMC 3284097
 . PMID 22310821.
. PMID 22310821. - ↑ "Summary of recommendations on when to start ART in children" (PDF). Consolidated ARV guidelines, June 2013. June 2013.
- ↑ "Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection" (PDF). Department of Health and Human Services, February 2014. March 2014.
- ↑ "Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings" (PDF). Department of HIV/AIDS, World Health Organization 2011. 2011.
- ↑ Laurence J (2006). "Hepatitis A and B virus immunization in HIV-infected persons". AIDS Reader. 16 (1): 15–17. PMID 16433468.
- 1 2 UNAIDS 2011 pg. 150–160
- ↑ Huang, L; Cattamanchi, A; Davis, JL; den Boon, S; Kovacs, J; Meshnick, S; Miller, RF; Walzer, PD; Worodria, W; Masur, H; International HIV-associated Opportunistic Pneumonias (IHOP), Study; Lung HIV, Study (June 2011). "HIV-associated Pneumocystis pneumonia". Proceedings of the American Thoracic Society. 8 (3): 294–300. doi:10.1513/pats.201009-062WR. PMC 3132788
 . PMID 21653531.
. PMID 21653531. - ↑ "Treating opportunistic infections among HIV-infected adults and adolescents. Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association/Infectious Diseases Society of America.". Department of Health and Human Services. February 2, 2007.
- 1 2 Smith, [edited by] Blaine T. (2008). Concepts in immunology and immunotherapeutics (4th ed.). Bethesda, Md.: American Society of Health-System Pharmacists. p. 143. ISBN 978-1-58528-127-5.
- 1 2 World Health Organization (May 2003). Nutrient requirements for people living with HIV/AIDS: Report of a technical consultation (PDF). Geneva. Archived (PDF) from the original on March 25, 2009. Retrieved March 31, 2009.
- 1 2 3 Irlam, JH; Visser, MM; Rollins, NN; Siegfried, N (December 8, 2010). Irlam, James H, ed. "Micronutrient supplementation in children and adults with HIV infection". Cochrane database of systematic reviews (Online) (12): CD003650. doi:10.1002/14651858.CD003650.pub3. PMID 21154354.
- ↑ Stone, CA; Kawai, K; Kupka, R; Fawzi, WW (November 2010). "Role of selenium in HIV infection". Nutrition Reviews. 68 (11): 671–81. doi:10.1111/j.1753-4887.2010.00337.x. PMC 3066516
 . PMID 20961297.
. PMID 20961297. - ↑ Forrester, JE; Sztam, KA (December 2011). "Micronutrients in HIV/AIDS: is there evidence to change the WHO 2003 recommendations?". The American Journal of Clinical Nutrition. 94 (6): 1683S–1689S. doi:10.3945/ajcn.111.011999. PMC 3226021
 . PMID 22089440.
. PMID 22089440. - ↑ Nunnari G, Coco C, Pinzone MR, Pavone P, Berretta M, Di Rosa M, Schnell M, Calabrese G, Cacopardo B (2012). "The role of micronutrients in the diet of HIV-1-infected individuals". Front Biosci (Elite Ed). 4: 2442–56. PMID 22652651.
- ↑ Zeng L, Zhang L (2011). "Efficacy and safety of zinc supplementation for adults, children and pregnant women with HIV infection: systematic review". Trop. Med. Int. Health. 16 (12): 1474–82. doi:10.1111/j.1365-3156.2011.02871.x. PMID 21895892.
- ↑ Littlewood RA, Vanable PA (September 2008). "Complementary and alternative medicine use among HIV-positive people: research synthesis and implications for HIV care". AIDS Care. 20 (8): 1002–18. doi:10.1080/09540120701767216. PMC 2570227
 . PMID 18608078.
. PMID 18608078. - ↑ Mills E, Wu P, Ernst E (June 2005). "Complementary therapies for the treatment of HIV: in search of the evidence". Int J STD AIDS. 16 (6): 395–403. doi:10.1258/0956462054093962. PMID 15969772.
- ↑ Liu JP, Manheimer E, Yang M (2005). Liu, Jian Ping, ed. "Herbal medicines for treating HIV infection and AIDS". Cochrane Database Syst Rev (3): CD003937. doi:10.1002/14651858.CD003937.pub2. PMID 16034917.
- ↑ Lutge EE, Gray A, Siegfried N (2013). "The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS". Cochrane Database Syst Rev. 4 (4): CD005175. doi:10.1002/14651858.CD005175.pub3. PMID 23633327.
- 1 2 3 Knoll B, Lassmann B, Temesgen Z (2007). "Current status of HIV infection: a review for non-HIV-treating physicians". Int J Dermatol. 46 (12): 1219–28. doi:10.1111/j.1365-4632.2007.03520.x. PMID 18173512.
- 1 2 Morgan D, Mahe C, Mayanja B, Okongo JM, Lubega R, Whitworth JA (2002). "HIV-1 infection in rural Africa: is there a difference in median time to AIDS and survival compared with that in industrialized countries?". AIDS. 16 (4): 597–632. doi:10.1097/00002030-200203080-00011. PMID 11873003.
- ↑ Zwahlen M, Egger M (2006). "Progression and mortality of untreated HIV-positive individuals living in resource-limited settings: update of literature review and evidence synthesis" (PDF). UNAIDS Obligation HQ/05/422204. Archived (PDF) from the original on April 9, 2008. Retrieved March 19, 2008.
- 1 2 Antiretroviral Therapy Cohort Collaboration (2008). "Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies". Lancet. 372 (9635): 293–9. doi:10.1016/S0140-6736(08)61113-7. PMC 3130543
 . PMID 18657708.
. PMID 18657708. - ↑ Schackman BR, Gebo KA, Walensky RP, Losina E, Muccio T, Sax PE, Weinstein MC, Seage GR 3rd, Moore RD, Freedberg KA. (2006). "The lifetime cost of current HIV care in the United States". Med Care. 44 (11): 990–997. doi:10.1097/01.mlr.0000228021.89490.2a. PMID 17063130.
- ↑ van Sighem, AI; Gras, LA; Reiss, P; Brinkman, K; de Wolf, F; ATHENA national observational cohort, study (June 19, 2010). "Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals". AIDS (London, England). 24 (10): 1527–35. doi:10.1097/QAD.0b013e32833a3946. PMID 20467289.
- 1 2 Cheung, MC; Pantanowitz, L; Dezube, BJ (Jun–Jul 2005). "AIDS-related malignancies: emerging challenges in the era of highly active antiretroviral therapy". The oncologist. 10 (6): 412–26. doi:10.1634/theoncologist.10-6-412. PMID 15967835.
- ↑ Tang J, Kaslow RA (2003). "The impact of host genetics on HIV infection and disease progression in the era of highly active antiretroviral therapy". AIDS. 17 (Suppl 4): S51–S60. doi:10.1097/00002030-200317004-00006. PMID 15080180.
- ↑ Lawn SD (2004). "AIDS in Africa: the impact of co-infections on the pathogenesis of HIV-1 infection". J. Infect. Dis. 48 (1): 1–12. doi:10.1016/j.jinf.2003.09.001. PMID 14667787.
- ↑ Campbell GR, Pasquier E, Watkins J, et al. (2004). "The glutamine-rich region of the HIV-1 Tat protein is involved in T-cell apoptosis". J. Biol. Chem. 279 (46): 48197–48204. doi:10.1074/jbc.M406195200. PMID 15331610.
- ↑ Campbell GR, Watkins JD, Esquieu D, Pasquier E, Loret EP, Spector SA (2005). "The C terminus of HIV-1 Tat modulates the extent of CD178-mediated apoptosis of T cells". J. Biol. Chem. 280 (46): 38376–39382. doi:10.1074/jbc.M506630200. PMID 16155003.
- ↑ "Tuberculosis". Fact sheet 104. World Health Organization. March 2012. Retrieved August 29, 2012.
- ↑ World Health Organization (2011). "Global tuberculosis control 2011" (PDF). ISBN 978-92-4-156438-0. Retrieved August 29, 2012.
- ↑ Pennsylvania, Editors, Raphael Rubin, M.D., Professor of Pathology, David S. Strayer, M.D., Ph.D., Professor of Pathology, Department of Pathology and Cell Biology, Jefferson Medical College of Thomas Jefferson University Philadelphia, Pennsylvania ; Founder and Consulting Editor, Emanuel Rubin, M.D., Gonzalo Aponte Distinguished Professor of Pathology, Chairman Emeritus of the Department of Pathology and Cell Biology, Jefferson Medical College of Thomas Jefferson University, Philadelphia, (2011). Rubin's pathology : clinicopathologic foundations of medicine (Sixth ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 154. ISBN 978-1-60547-968-2.
- ↑ Woods, S.; Moore, D.; Weber, E.; Grant, I. (2009). "Cognitive neuropsychology of HIV-associated neurocognitive disorders". Neuropsychology review. 19 (2): 152–168. doi:10.1007/s11065-009-9102-5. PMC 2690857
 . PMID 19462243.
. PMID 19462243. - ↑ Brown, T.; Qaqish, R. (2006). "Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review". AIDS (London, England). 20 (17): 2165–2174. doi:10.1097/QAD.0b013e32801022eb. PMID 17086056.
- ↑ Nicholas, P.K.; Kemppainen, J.K.; Canaval, G.E.; et al. (February 2007). "Symptom management and self-care for peripheral neuropathy in HIV/AIDS". AIDS Care. 19 (2): 179–89. doi:10.1080/09540120600971083. PMID 17364396.
- ↑ Boshoff C, Weiss R (2002). "AIDS-related malignancies". Nature Reviews Cancer. 2 (5): 373–382. doi:10.1038/nrc797. PMID 12044013.
- ↑ Yarchoan R, Tosato G, Little RF (2005). "Therapy insight: AIDS-related malignancies – the influence of antiviral therapy on pathogenesis and management". Nat. Clin. Pract. Oncol. 2 (8): 406–415. doi:10.1038/ncponc0253. PMID 16130937.
- ↑ Post, F. .; Holt, S. . (2009). "Recent developments in HIV and the kidney". Current opinion in infectious diseases. 22 (1): 43–48. doi:10.1097/QCO.0b013e328320ffec. PMID 19106702.
- ↑ "AIDSinfo". UNAIDS. Retrieved March 4, 2013.
- ↑ Cohen, MS; Hellmann, N; Levy, JA; DeCock, K; Lange, J (April 2008). "The spread, treatment, and prevention of HIV-1: evolution of a global pandemic". The Journal of Clinical Investigation. 118 (4): 1244–54. doi:10.1172/JCI34706. PMC 2276790
 . PMID 18382737.
. PMID 18382737. - 1 2 3 "Fact sheet 2015" (PDF). UNAIDS. Retrieved 1 February 2016.
- 1 2 "UNAIDS reports a 52% reduction in new HIV infections among children and a combined 33% reduction among adults and children since 2001". UNAIDS. Retrieved October 7, 2013.
- ↑ "Statistics: Women and HIV/AIDS". amfAR. July 2015. Retrieved 1 February 2016.
- 1 2 3 4 UNAIDS 2011 pg. 20–30
- 1 2 3 UNAIDS 2011 pg. 40–50
- 1 2 3 4 Mandell, Bennett, and Dolan (2010). Chapter 117.
- ↑ New HIV infections among children have been reduced by 50% or more in seven countries in sub-Saharan Africa, UN AIDS, Geneva, June 25, 2013.
- ↑ Centers for Disease Control and Prevention, (CDC) (June 3, 2011). "HIV surveillance—United States, 1981–2008". MMWR. Morbidity and mortality weekly report. 60 (21): 689–93. PMID 21637182.
- ↑ Health Protection Agency (2010). HIV in the United Kingdom: 2010 Report.
- ↑ Surveillance; riques, Risk Assessment Division = Le VIH et le sida au Canada: rapport de surveillance en date du 31 décembre 2009 / Division de la surveillance et de l'évaluation des (2010). HIV and AIDS in Canada : surveillance report to December 31, 2009 (PDF). Ottawa: Public Health Agency of Canada, Centre for Communicable Diseases and Infection Control, Surveillance and Risk Assessment Division. ISBN 978-1-100-52141-1.
- ↑ "Global Report Fact Sheet" (PDF). UNAIDS. 2010.
- ↑ "COUNTRY COMPARISON :: HIV/AIDS – ADULT PREVALENCE RATE". CIA World Factbook. Retrieved November 6, 2014.
- ↑ Gottlieb MS (2006). "Pneumocystis pneumonia—Los Angeles. 1981". Am J Public Health. 96 (6): 980–1; discussion 982–3. doi:10.2105/AJPH.96.6.980. PMC 1470612
 . PMID 16714472. Archived from the original on April 22, 2009. Retrieved March 31, 2009.
. PMID 16714472. Archived from the original on April 22, 2009. Retrieved March 31, 2009. - ↑ Friedman-Kien AE (October 1981). "Disseminated Kaposi's sarcoma syndrome in young homosexual men". J. Am. Acad. Dermatol. 5 (4): 468–71. doi:10.1016/S0190-9622(81)80010-2. PMID 7287964.
- ↑ Hymes KB, Cheung T, Greene JB, et al. (September 1981). "Kaposi's sarcoma in homosexual men-a report of eight cases". Lancet. 2 (8247): 598–600. doi:10.1016/S0140-6736(81)92740-9. PMID 6116083.
- 1 2 Basavapathruni, A; Anderson, KS (December 2007). "Reverse transcription of the HIV-1 pandemic". The FASEB Journal. 21 (14): 3795–3808. doi:10.1096/fj.07-8697rev. PMID 17639073.
- ↑ Centers for Disease Control (CDC) (1982). "Persistent, generalized lymphadenopathy among homosexual males". MMWR Morb Mortal Wkly Rep. 31 (19): 249–251. PMID 6808340. Retrieved August 31, 2011.
- ↑ Barré-Sinoussi, F.; Chermann, J.C.; Rey, F.; et al. (1983). "Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS)". Science. 220 (4599): 868–871. Bibcode:1983Sci...220..868B. doi:10.1126/science.6189183. PMID 6189183.
- 1 2 Centers for Disease Control (CDC) (1982). "Opportunistic infections and Kaposi's sarcoma among Haitians in the United States". MMWR Morb Mortal Wkly Rep. 31 (26): 353–354; 360–361. PMID 6811853. Retrieved August 31, 2011.
- ↑ Gilman, Sander L., ed. (1987). "AIDS and Syphilis: The Iconography of Disease". Retrieved April 25, 2015.
- ↑ "Making Headway Under Hellacious Circumstances" (PDF). American Association for the Advancement of Science. July 28, 2006. Retrieved June 23, 2008.
- ↑ Altman LK (May 11, 1982). "New homosexual disorder worries health officials". The New York Times. Retrieved August 31, 2011.
- ↑ Kher U (July 27, 1982). "A Name for the Plague". Time. Archived from the original on March 7, 2008. Retrieved March 10, 2008.
- ↑ Centers for Disease Control (CDC) (1982). "Update on acquired immune deficiency syndrome (AIDS)—United States". MMWR Morb Mortal Wkly Rep. 31 (37): 507–508; 513–514. PMID 6815471.
- ↑ RC Gallo; PS Sarin; EP Gelmann; M Robert-Guroff; E Richardson; VS Kalyanaraman; D Mann; GD Sidhu; RE Stahl; S Zolla-Pazner; J Leibowitch; M Popovic (1983). "Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS)". Science. 220 (4599): 865–867. Bibcode:1983Sci...220..865G. doi:10.1126/science.6601823. PMID 6601823.
- ↑ Barre-Sinoussi, F.; Chermann, J.; Rey, F.; Nugeyre, M.; Chamaret, S.; Gruest, J.; Dauguet, C.; Axler-Blin, C.; Vézinet-Brun, F.; Rouzioux, C.; Rozenbaum, W.; Montagnier, L. (1983). "Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS)". Science. 220 (4599): 868–871. Bibcode:1983Sci...220..868B. doi:10.1126/science.6189183. PMID 6189183.
- ↑ Aldrich, ed. by Robert; Wotherspoon, Garry (2001). Who's who in gay and lesbian history. London: Routledge. p. 154. ISBN 978-0-415-22974-6.
- ↑ Gao, F.; Bailes, E.; Robertson, D.L.; et al. (February 1999). "Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes". Nature. 397 (6718): 436–41. Bibcode:1999Natur.397..436G. doi:10.1038/17130. PMID 9989410.
- ↑ Keele, B. F., van Heuverswyn, F., Li, Y. Y., Bailes, E., Takehisa, J., Santiago, M. L., Bibollet-Ruche, F., Chen, Y., Wain, L. V., Liegois, F., Loul, S., Mpoudi Ngole, E., Bienvenue, Y., Delaporte, E., Brookfield, J. F. Y., Sharp, P. M., Shaw, G. M., Peeters, M., and Hahn, B. H. (July 28, 2006). "Chimpanzee Reservoirs of Pandemic and Nonpandemic HIV-1". Science. 313 (5786): 523–6. Bibcode:2006Sci...313..523K. doi:10.1126/science.1126531. PMC 2442710
 . PMID 16728595.
. PMID 16728595. - ↑ Goodier, J.; Kazazian, H. (2008). "Retrotransposons Revisited: The Restraint and Rehabilitation of Parasites". Cell. 135 (1): 23–35. doi:10.1016/j.cell.2008.09.022. PMID 18854152.(subscription required)
- ↑ Sharp, P. M.; Bailes, E.; Chaudhuri, R. R.; Rodenburg, C. M.; Santiago, M. O.; Hahn, B. H. (2001). "The origins of acquired immune deficiency syndrome viruses: where and when?" (PDF). Philosophical Transactions of the Royal Society B. 356 (1410): 867–76. doi:10.1098/rstb.2001.0863. PMC 1088480
 . PMID 11405934.
. PMID 11405934. - ↑ Kalish, M.; Wolfe, N.D.; Ndongmo, C.D.; McNicholl. J.; Robbins, K.E.; et al. (2005). "Central African hunters exposed to simian immunodeficiency virus". Emerg Infect Dis. 11 (12): 1928–30. doi:10.3201/eid1112.050394. PMC 3367631
 . PMID 16485481.
. PMID 16485481. - 1 2 Marx PA, Alcabes PG, Drucker E (2001). "Serial human passage of simian immunodeficiency virus by unsterile injections and the emergence of epidemic human immunodeficiency virus in Africa" (PDF). Philosophical Transactions of the Royal Society B. 356 (1410): 911–20. doi:10.1098/rstb.2001.0867. PMC 1088484
 . PMID 11405938.
. PMID 11405938. - ↑ Worobey, Michael; Gemmel, Marlea; Teuwen, Dirk E.; Haselkorn, Tamara; Kunstman, Kevin; Bunce, Michael; Muyembe, Jean-Jacques; Kabongo, Jean-Marie M.; Kalengayi, Raphaël M.; Van Marck, Eric; Gilbert, M. Thomas P.; Wolinsky, Steven M. (2008). "Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960" (PDF). Nature. 455 (7213): 661–4. Bibcode:2008Natur.455..661W. doi:10.1038/nature07390. PMC 3682493
 . PMID 18833279. (subscription required)
. PMID 18833279. (subscription required) - 1 2 Sousa, João Dinis de; Müller, Viktor; Lemey, Philippe; Vandamme, Anne-Mieke; Vandamme, Anne-Mieke (2010). Martin, Darren P., ed. "High GUD Incidence in the Early 20th Century Created a Particularly Permissive Time Window for the Origin and Initial Spread of Epidemic HIV Strains". PLoS ONE. 5 (4): e9936. doi:10.1371/journal.pone.0009936. PMC 2848574
 . PMID 20376191.
. PMID 20376191. - ↑ Chitnis, Amit; Rawls, Diana; Moore, Jim (2000). "Origin of HIV Type 1 in Colonial French Equatorial Africa?". AIDS Research and Human Retroviruses. 16 (1): 5–8. doi:10.1089/088922200309548. PMID 10628811.(subscription required)
- ↑ Donald G. McNeil, Jr. (September 16, 2010). "Precursor to H.I.V. Was in Monkeys for Millennia". New York Times. Retrieved September 17, 2010.
Dr. Marx believes that the crucial event was the introduction into Africa of millions of inexpensive, mass-produced syringes in the 1950s. ... suspect that the growth of colonial cities is to blame. Before 1910, no Central African town had more than 10,000 people. But urban migration rose, increasing sexual contacts and leading to red-light districts.
- ↑ Zhu, T., Korber, B. T., Nahmias, A. J., Hooper, E., Sharp, P. M. and Ho, D. D. (1998). "An African HIV-1 Sequence from 1959 and Implications for the Origin of the epidemic". Nature. 391 (6667): 594–7. Bibcode:1998Natur.391..594Z. doi:10.1038/35400. PMID 9468138.
- ↑ Lederberg, editor-in-chief Joshua (2000). Encyclopedia of Microbiology, (4 Volume Set). (2nd ed.). Burlington: Elsevier. p. 106. ISBN 9780080548487. Retrieved 9 June 2016.
- ↑ Jackson, Regine O., ed. (2011). "Geographies of the Haitian Diaspora". Routledge. p. 12. ISBN 9780415887083. Retrieved 13 March 2016.
- 1 2 Pépin, Jacques (2011). "The Origin of Aids". Cambridge University Press. p. 188. ISBN 9780521186377. Retrieved 13 March 2016.
- ↑ Kolata, Gina (October 28, 1987). "Boy's 1969 Death Suggests AIDS Invaded U.S. Several Times". The New York Times. Retrieved February 11, 2009.
- 1 2 Gilbert, M. Thomas P.; Rambaut, Andrew; Wlasiuk, Gabriela; Spira, Thomas J.; Pitchenik, Arthur E.; Worobey, Michael (November 20, 2007). "The emergence of HIV/AIDS in the Americas and beyond" (PDF). PNAS. 104 (47): 18566–18570. Bibcode:2007PNAS..10418566G. doi:10.1073/pnas.0705329104. PMC 2141817
 . PMID 17978186.
. PMID 17978186. - ↑ "Ryan White, an American AIDS Victim". Encyclopædia Britannica. November 7, 2013. Retrieved July 16, 2015.
- ↑ Ogden J, Nyblade L (2005). "Common at its core: HIV-related stigma across contexts" (PDF). International Center for Research on Women. Retrieved February 15, 2007.
- 1 2 3 Herek GM, Capitanio JP (1999). "AIDS Stigma and sexual prejudice" (PDF). American Behavioral Scientist. 42 (7): 1130–1147. doi:10.1177/0002764299042007006. Retrieved March 27, 2006.
- ↑ Snyder M, Omoto AM, Crain AL (1999). "Punished for their good deeds: stigmatization for AIDS volunteers". American Behavioral Scientist. 42 (7): 1175–1192. doi:10.1177/0002764299042007009.
- ↑ Sharma, A.K. (2012). Population and society. New Delhi: Concept Pub. Co. p. 242. ISBN 978-81-8069-818-7.
- ↑ Herek, GM; Capitanio, JP; Widaman, KF (March 2002). "HIV-related stigma and knowledge in the United States: prevalence and trends, 1991–1999". American Journal of Public Health. 92 (3): 371–7. doi:10.2105/AJPH.92.3.371. PMC 1447082
 . PMID 11867313.
. PMID 11867313. - ↑ De Cock, KM; Jaffe, HW; Curran, JW (June 19, 2012). "The evolving epidemiology of HIV/AIDS". AIDS (London, England). 26 (10): 1205–13. doi:10.1097/QAD.0b013e328354622a. PMID 22706007.
- ↑ Richard Spencer (August 21, 2003). "China relaxes laws on love and marriage". The Telegraph. Retrieved October 24, 2013.
- ↑ Bell C, Devarajan S, Gersbach H (2003). "The long-run economic costs of AIDS: theory and an application to South Africa" (PDF). World Bank Policy Research Working Paper No. 3152. Retrieved April 28, 2008.
- 1 2 Greener R (2002). "AIDS and macroeconomic impact". In S, Forsyth. State of The Art: AIDS and Economics (PDF). IAEN. pp. 49–55.
- ↑ Robinson, Rachel; Okpo, Emmanuel; Mngoma, Nomusa (2015). "Interventions for improving employment outcomes for workers with HIV". The Cochrane Database of Systematic Reviews. 5: CD010090. doi:10.1002/14651858.CD010090.pub2. ISSN 1469-493X. PMID 26022149.
- ↑ Over M (1992). "The macroeconomic impact of AIDS in Sub-Saharan Africa, Population and Human Resources Department" (PDF). The World Bank. Archived (PDF) from the original on May 27, 2008. Retrieved May 3, 2008.
- ↑ "AIDS Stigma". News-medical.net. Retrieved November 1, 2011.
- 1 2 "Thirty years after AIDS discovery, appreciation growing for Catholic approach". Catholicnewsagency.com. June 5, 2011. Retrieved November 1, 2011.
- 1 2 "Church HIV prayer cure claims 'cause three deaths'". BBC News. October 18, 2011. Retrieved October 18, 2011.
- ↑ "Rock Hudson announces he has AIDS – History.com This Day in History – 7/25/1985". History.com. Retrieved November 1, 2011.
- ↑ Coleman, Brian (June 25, 2007). "Thatcher the gay icon". New Statesman. Retrieved November 1, 2011.
- ↑ "November 24, 1991: Giant of rock dies". BBC On This Day. BBC News. November 24, 1991. Archived from the original on October 21, 2011. Retrieved November 1, 2011.
- ↑ "Freddie Mercury". Nndb.com. Retrieved November 1, 2011.
- ↑ Bliss, Dominic. "Frozen In Time: Arthur Ashe". iTENNISstore.com. Retrieved June 25, 2012.
- ↑ "Tributes to Arthur Ashe". The Independent. London. February 8, 1993. Retrieved July 24, 2012.
- ↑ Cosgrove, Ben. "Behind the Picture: The Photo That Changed the Face of AIDS". LIFE magazine. Retrieved August 16, 2012.
- ↑ "Aziga found guilty of first-degree murder". CTV.ca News. Retrieved April 9, 2013.
- ↑ "HIV killer ruled dangerous offender". CBC News. Retrieved April 9, 2013.
- ↑ "A fraudster, not a murderer". National Post. Retrieved April 9, 2013.
- ↑ "HIV-Specific Criminal Laws". cdc.gov. June 30, 2014. Retrieved November 22, 2014.
- ↑ "'Virgin cure': Three women killed to 'cure' Aids". International Herald Tribune. February 28, 2013. Retrieved September 14, 2013.
- ↑ Jenny, Carole (2010). Child Abuse and Neglect: Diagnosis, Treatment and Evidence – Expert Consult. Elsevier Health Sciences. p. 187. ISBN 978-1-4377-3621-2.
- ↑ Klot, Jennifer; Monica Kathina Juma (2011). HIV/AIDS, Gender, Human Security and Violence in Southern Africa. Pretoria: Africa Institute of South Africa. p. 47. ISBN 0-7983-0253-4.
- ↑ "HIV Public Knowledge and Attitudes 2014" (pdf). National AIDS Trust. Nov 2014. p. 9. Retrieved February 12, 2015.
- ↑ Blechner MJ (1997). Hope and mortality: psychodynamic approaches to AIDS and HIV. Hillsdale, NJ: Analytic Press. ISBN 0-88163-223-6.
- ↑ Kirby DB, Laris BA, Rolleri LA (March 2007). "Sex and HIV education programs: their impact on sexual behaviors of young people throughout the world". J Adolesc Health. 40 (3): 206–17. doi:10.1016/j.jadohealth.2006.11.143. PMID 17321420.
- ↑ Duesberg, P. H. (1988). "HIV is not the cause of AIDS". Science. 241 (4865): 514, 517. Bibcode:1988Sci...241..514D. doi:10.1126/science.3399880. PMID 3399880.Cohen, J. (1994). "The Controversy over HIV and AIDS" (PDF). Science. 266 (5191): 1642–1649. Bibcode:1994Sci...266.1642C. doi:10.1126/science.7992043. PMID 7992043. Retrieved March 31, 2009.
- ↑ Kalichman, Seth (2009). Denying AIDS: Conspiracy Theories, Pseudoscience, and Human Tragedy. New York: Copernicus Books (Springer Science+Business Media). ISBN 978-0-387-79475-4.
- ↑ Smith TC, Novella SP (August 2007). "HIV Denial in the Internet Era". PLoS Med. 4 (8): e256. doi:10.1371/journal.pmed.0040256. PMC 1949841
 . PMID 17713982. Retrieved November 7, 2009.
. PMID 17713982. Retrieved November 7, 2009. - ↑ Various (January 14, 2010). "Resources and Links, HIV-AIDS Connection". National Institute of Allergy and Infectious Diseases. Retrieved February 22, 2009.
- ↑ Watson J (2006). "Scientists, activists sue South Africa's AIDS 'denialists'". Nat. Med. 12 (1): 6. doi:10.1038/nm0106-6a. PMID 16397537.
- ↑ Baleta A (2003). "S Africa's AIDS activists accuse government of murder". Lancet. 361 (9363): 1105. doi:10.1016/S0140-6736(03)12909-1. PMID 12672319.
- ↑ Cohen J (2000). "South Africa's new enemy". Science. 288 (5474): 2168–70. doi:10.1126/science.288.5474.2168. PMID 10896606.
- ↑ Boghardt, Thomas (2009). "Operation INFEKTION Soviet Bloc Intelligence and Its AIDS Disinformation Campaign". Central Intelligence Agency.
Further reading
- Mandell, Gerald L.; Bennett, John E.; Dolin, Raphael, eds. (2010). Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases (7th ed.). Philadelphia, PA: Churchill Livingstone/Elsevier. ISBN 978-0-443-06839-3.
- Joint United Nations Programme on HIV/AIDS (UNAIDS) (2011). Global HIV/AIDS Response, Epidemic update and health sector progress towards universal access (PDF). Joint United Nations Programme on HIV/AIDS.
External links
| Wikimedia Commons has media related to AIDS. |
- HIV/AIDS at DMOZ.
- UNAIDS – Joint United Nations Program on HIV/AIDS.
- AIDSinfo – Information on HIV/AIDS treatment, prevention, and research, U.S. Department of Health and Human Services.