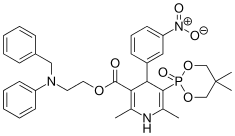
Efonidipine
 | |
| Clinical data | |
|---|---|
| Trade names | Landel (ランデル) |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
111011-63-3 |
| PubChem (CID) | 119171 |
| ChemSpider |
106463 |
| UNII |
40ZTP2T37Q |
| ChEMBL |
CHEMBL2074922 |
| Chemical and physical data | |
| Formula | C34H38N3O7P |
| Molar mass | 631.65 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Efonidipine (INN) is a dihydropyridine calcium channel blocker marketed by Shionogi & Co. of Japan. It was launched in 1995, under the brand name Landel (ランデル). The drug blocks both T-type and L-type calcium channels.[1]
It has also been studied in atherosclerosis[2] and acute renal failure.[3]
References
- ↑ Tanaka H, Shigenobu K (2002). "Efonidipine hydrochloride: a dual blocker of L- and T-type Ca2+ channels". Cardiovasc. Drug Rev. 20 (1): 81–92. PMID 12070536.
- ↑ Toyoda K, Kitahara M, Yamashita T, et al. (May 1994). "[Effect of efonidipine hydrochloride (NZ-105), a new dihydropyridine calcium antagonist, on the experimental atherosclerosis in cholesterol-fed rabbits]". Nippon Yakurigaku Zasshi (in Japanese). 103 (5): 231–9. PMID 8188119.
- ↑ Shudo C, Masuda Y, Sugita H, Tanaka S, Tomita K (November 1994). "Effects of efonidipine hydrochloride (NZ-105), a new calcium antagonist, against acute renal failure in rats". Gen. Pharmacol. 25 (7): 1451–8. PMID 7896060.
- (Japanese) Landel ランデル (PDF) Shionogi & Co. April 2005.
This article is issued from Wikipedia - version of the 8/12/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.