PRL-8-53
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
51352-88-6 51352-87-5 (HCl) |
| PubChem (CID) | 39988 |
| ChemSpider | 36560 |
| Chemical and physical data | |
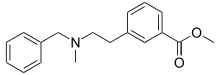
| Formula | C18H21NO2 |
| Molar mass | 283.36 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
PRL-8-53 is a nootropic research chemical derived from benzoic acid and phenylmethylamine (Benzylamine) that has been shown to act as a hypermnesic drug in humans; it was first synthesized by medical chemistry professor Nikolaus Hansl at Creighton University in the 1970s as part of his work on amino ethyl meta benzoic acid esters.[1][2]
Nootropic effects
A single study in humans was reported in 1978. The double-blind trial of PRL-8-53 in 47 healthy volunteers measured its effects on a variety of cognitive measures. 5 mg of the drug was administered orally 2–2.5 hours before the study tasks.[1] Overall improvements in recollection differed based on how many words were recalled under placebo, with the poor performers (six words or fewer) experiencing a 87.5-105% increase in recollection and the high performers (eight or more words) a 7.9-14% increase which failed to reach statistical significance; when controlling for subjects over the age of 30 only, a 108-152% increase was noted..[1] No side effects were reported during the trial.[1]
Mechanism of action
The exact mechanism of action of PRL-8-53 remains unknown. Doses up to 200 mg/kg are not observed to have stimulant properties, and a dosage of 20 mg/kg does not potentiate the effects of dextroamphetamine in rats.[1] It displays possible cholinergic properties, and potentiates dopamine while partially inhibiting serotonin. PRL-8-53 reverses the catatonic and ptotic effects of reserpine.[1][3]
Toxicity
PRL-8-53 is relatively non-toxic, with an oral LD50 in mice of 860 mg/kg, giving the drug a high therapeutic index. Doses above 8 mg/kg have brief hypotensive effects in the canine. High doses depress motor activity in the rat and mouse, with the ED50 for a 50% reduction in motor activity of mice at 160 mg/kg. PRL-8-53 displays spasmolytic effects.[3]
Synonyms
Methyl 3-(2-(benzylmethylamino)ethyl)benzoate hydrochloride
3-(2-benzylmethylaminoethyl) benzoic acid methyl ester hydrochloride
3-(2-(Methyl(phenylmethyl)amino)ethyl)benzoic acid methyl ester hydrochloride
See also
References
- 1 2 3 4 5 6 Hansl, NR; Nikolaus R. Hansl, Beverley T. Mead (1978). "PRL-8-53: Enhanced learning and subsequent retention in humans as a result of low oral doses of new psychotropic agent". Psychopharmacology. 56 (3): 249–253. doi:10.1007/BF00432846. PMID 418433. Cite uses deprecated parameter
|coauthors=(help) - ↑ US Patent 3870715 A: Substituted amino ethyl meta benzoic acid esters
- 1 2 Hansl, N. R. (1974). "A novel spasmolytic and CNS active agent: 3-(2-benzylmethylamino ethyl) benzoic acid methyl ester hydrochloride". Experientia. 30 (3): 271–272. doi:10.1007/BF01934822. PMID 4824605.