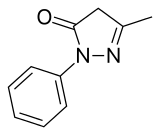
Edaravone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Radicut |
| Routes of administration | Oral |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms | MCI-186 |
| CAS Number |
89-25-8 |
| PubChem (CID) | 4021 |
| ChemSpider |
3881 |
| UNII |
S798V6YJRP |
| KEGG |
D01552 |
| ChEBI |
CHEBI:31530 |
| ChEMBL |
CHEMBL290916 |
| ECHA InfoCard | 100.001.719 |
| Chemical and physical data | |
| Formula | C10H10N2O |
| Molar mass | 174.20 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Edaravone (brand name ラジカット, Radicut) is a nootropic and neuroprotective agent used for the purpose of aiding neurological recovery following acute brain ischemia and subsequent cerebral infarction.[1] It acts as a potent antioxidant and strongly scavenges free radicals, protecting against oxidative stress and neuronal apoptosis.[2][3][4] It has been marketed solely in Japan by Mitsubishi Pharma since 2001.[1] It is also marketed in India by Edinburgh Pharmaceuticals by the brand name Arone.
On June 26, 2015, Mitsubishi Tanabe Pharma Corporation announced it has received approval to market Radicut for treatment of ALS in Japan. The phase III clinical trial began in 2011 in Japan. The company was awarded Orphan Drug Designation for Radicut by the FDA and EU in 2015. Radicut is an intravenous drug and administrated 14 days followed by 14 days drug holiday.
The biotech company Treeway is developing an oral formulation of edaravone (TW001) and is currently in clinical development. Treeway was awarded orphan drug designation for edaravone by the EMA in November 2014 and FDA in January 2015.
Edaravone has been shown to attenuate methamphetamine- and 6-OHDA-induced dopaminergic neurotoxicity in the striatum and substantia nigra, and does not affect methamphetamine-induced dopamine release or hyperthermia.[5][6] It has also been demonstrated to protect against MPTP-mediated dopaminergic neurotoxicity to the substantia nigra, though notably not to the striatum.[7][8][9]
References
- 1 2 Doherty, Annette M. (2002). Annual Reports in Medicinal Chemistry, Volume 37 (Annual Reports in Medicinal Chemistry). Boston: Academic Press. ISBN 0-12-040537-7.
- ↑ Watanabe T, Tanaka M, Watanabe K, Takamatsu Y, Tobe A (March 2004). "[Research and development of the free radical scavenger edaravone as a neuroprotectant]". Yakugaku Zasshi (in Japanese). 124 (3): 99–111. doi:10.1248/yakushi.124.99. PMID 15049127.
- ↑ Higashi Y, Jitsuiki D, Chayama K, Yoshizumi M (January 2006). "Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a novel free radical scavenger, for treatment of cardiovascular diseases". Recent Patents on Cardiovascular Drug Discovery. 1 (1): 85–93. doi:10.2174/157489006775244191. PMID 18221078.
- ↑ Yoshida H, Yanai H, Namiki Y, Fukatsu-Sasaki K, Furutani N, Tada N (2006). "Neuroprotective effects of edaravone: a novel free radical scavenger in cerebrovascular injury". CNS Drug Reviews. 12 (1): 9–20. doi:10.1111/j.1527-3458.2006.00009.x. PMID 16834755.
- ↑ Yuan WJ, Yasuhara T, Shingo T, et al. (2008). "Neuroprotective effects of edaravone-administration on 6-OHDA-treated dopaminergic neurons". BMC Neuroscience. 9: 75. doi:10.1186/1471-2202-9-75. PMC 2533664
 . PMID 18671880.
. PMID 18671880. - ↑ Kawasaki T, Ishihara K, Ago Y, et al. (August 2006). "Protective effect of the radical scavenger edaravone against methamphetamine-induced dopaminergic neurotoxicity in mouse striatum". European Journal of Pharmacology. 542 (1-3): 92–9. doi:10.1016/j.ejphar.2006.05.012. PMID 16784740.
- ↑ Kawasaki T, Ishihara K, Ago Y, Baba A, Matsuda T (July 2007). "Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a radical scavenger, prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in the substantia nigra but not the striatum". The Journal of Pharmacology and Experimental Therapeutics. 322 (1): 274–81. doi:10.1124/jpet.106.119206. PMID 17429058.
- ↑ Yokoyama H, Takagi S, Watanabe Y, Kato H, Araki T (June 2008). "Role of reactive nitrogen and reactive oxygen species against MPTP neurotoxicity in mice". Journal of Neural Transmission (Vienna, Austria : 1996). 115 (6): 831–42. doi:10.1007/s00702-008-0019-6. PMID 18235988.
- ↑ Yokoyama H, Yano R, Aoki E, Kato H, Araki T (September 2008). "Comparative pharmacological study of free radical scavenger, nitric oxide synthase inhibitor, nitric oxide synthase activator and cyclooxygenase inhibitor against MPTP neurotoxicity in mice". Metabolic Brain Disease. 23 (3): 335–49. doi:10.1007/s11011-008-9096-3. PMID 18648914.
External links
- http://www.alstdi.org/news/radicut-approved-for-als-in-japan/
- http://www.mt-pharma.co.jp/e/release/nr/2015/pdf/e_MTPC150626_2.pdf
- http://www.alstdi.org/als-research/clinical-trials/168/
- http://informahealthcare.com/doi/pdfplus/10.3109/21678421.2014.959024