Heligmosomoides polygyrus
| Heligmosomoides polygyrus | |
|---|---|
 | |
| Female H. polygyrus from the digestive tract of a woodmouse | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Nematoda |
| Class: | Chromadorea |
| Order: | Rhabditida |
| Family: | Trychostrongylidae |
| Genus: | Heligmosomoides |
| Species: | H. polygyrus |
| Binomial name | |
| Heligmosomoides polygyrus | |
Heligmosomoides polygyrus, previously named Nematospiroides dubius, is a naturally occurring intestinal roundworm of rodents.[1] It belongs to the family Trychostrongylidae, and male and female worms are morphologically distinguishable.[2] The parasite has a direct life cycle with its larval form being the infective stage. H. polygyrus has the ability to establish chronic infections in rodents and alter host immune responses. This nematode is widely used as a gastrointestinal parasitic model in immunological, pharmacological and toxicological studies.[3]
Life cycle and morphology
This parasite has a direct life cycle with no intermediate hosts. The life cycle takes about 13–15 days to complete.[1][4] Infected mice will pass faeces containing eggs and egg sizes vary between 70–84 micrometres (µm) in length and 37–53 µm in width.[5] Eggs are shed from the host at the 8–16 cell stage and will hatch in the environment, roughly 24 hours after passing through the host.[6] L1 larvae will emerge from the egg and measure between 300–600 µm in length. Three lip-like structures can be seen around a rudimentary mouth. L1 larvae moult to L2 larvae after 2–3 days, entering bacterial-feeding larval stages present in the environment. The L1 stage cuticle will loosen from either end of the larvae but will remain loosely associated with the L2 larvae, becoming an outer sheath up until infection. After 3 days, the L2 partially moults into ensheathed L3, the infective non-feeding stage. Infective larval stages measure between 480–563 µm long.

Mice ingest the L3 stage of the parasite and after 18 hours, exsheathed L3 appears in the intestinal lumen. The L1 sheath is shed following ingestion at which point the larvae shorten slightly and measure between 376–540 µm in length. After 24 hours post ingestion, larvae will invade the mucosal layer of the intestine. After approximately 4 days post ingestion, L3 moult into L4 in the submucosa of the intestine. Approximately 6 days post ingestion they will encyst in the muscle layer of the intestine and starts maturing in to adult parasites. By day 14 post ingestion, adult male and female worms will come in to contact in the lumen of the intestine, mate and produce eggs that are passed in the faeces continuing the life cycle. Adult males are tightly coiled and usually measure 8–10mm in length (5). The females are also tightly coiled but larger, measuring between 18–21mm in length. Adults are characterized by a dark red pigmentation, whereas the free-living larval forms are mostly translucent.
Epidemiology
In natural infections, H. polygyrus is found almost ubiquitously within populations of wild wood mice (Apodemus sylvaticus). In one study of wood mouse populations in Oxfordshire, England, 70% of all mice sampled carried an infection with H. polygyrus, with an average infection burden of about 12 worms per mouse.[7] Natural infection intensity displays high variability in wood mice, ranging from 0 – 244 adult worms per mouse. Both male and female mice show equal parasitic burdens. Parasite occurrence appears to positively correlate with weight and age of the mouse, showing an increase in prevalence in older, heavier mice. Infection was also seasonally regulated in the wood mouse population, with highest prevalence of infection/worm burden intensity occurring in early spring and reaching their lowest values in late summer/early autumn. This is inversely correlated with typical breeding behaviors of the wood mouse, where the population peaks in late summer or early autumn, and is at its lowest in the early spring.[7] The bulk of research on H. polygyrus has been conducted on the laboratory mouse, Mus musculus, as it is used as a model of human helminth infection to which there is a spectrum of natural resistance to parasite infection.[4]
Pathogenicity
Upon infection with H. polygyrus, innate and adaptive host immune responses are generated to prevent the establishment of the parasite in the gut. A strong wound healing immune response (Th2-type) associated with intestinal pathology is mounted. Similar to other roundworm infections Th2 immunity focuses on eliminating the parasite or confining it to minimize host damage.
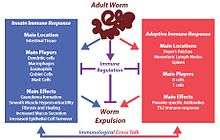
Mucus secreted by goblet cells of the intestine, acts as the first line of defense, hence increases in goblet cell number are a major observable change during H. polygyrus infection.[8] Macrophages are activated through Th2 cytokines and they are important in parasite clearance by increase intestinal motility and to induce fibrosis and healing.[9] These immune cells are also important in granuloma formation. This is a defensive response by the host to trap the parasite and minimize its damage to the gut. In addition, these cells are important in increasing contractions of the gut wall, which facilitates worm expulsion.[4] The spleen, mesenteric lymph nodes, Peyer’s patches and lamina propria lymphocytes induce a strong Th2 immune response by producing different cytokines (Interleukin 3, IL4, IL5, IL9, IL10 and IL13) which are important in controlling and expelling worms. These cytokines aid in generating CD4 T helper 2 effector cells necessary for adaptive immune responses against the parasite. In addition costimulatory signals via CD80 and CD86 has also be shown important in mounting a Th2 immune response and producing immunoglobulin E (IgE).[5] In the humoral arm of immunity, parasite specific IgG1 plays a greater role in protection during infection and IgA has been shown to have a minor effect. But IgM and IgE has not been shown important against H. polygyrus protection.
However, despite this impressive immune response, H. polygyrus is able to hijack the host immune response, dampening the Th2 response generated against itself, resulting in chronic infection. This immune regulation occurs through a strong Treg response elicited in the spleen and the mesenteric lymph nodes of the host, mainly involving CD25+CD103+ regulatory T cells.[10]
Prevention and treatment
No formal prevention strategies exist for control of H. polygyrus although the parasite is susceptible to a number of drug treatments. Treatment of an infected mouse with pyrantel pamoate, ivermectin or other anthelmintic drugs will help clear infection and provide immunity to reinfection.[4] Furthermore, a cocktail of H. polygyrus excretory-secretory antigens can be collected, and administered to mice in the presence of alum to induce sterilizing immunity pre-infection.[4][11]
Notes and references
- 1 2 Gregory, Richard D.; Keymer, Anne E.; Clarke, John R. (1990-01-01). "Genetics, Sex and Exposure: The Ecology of Heligmosomoides polygyrus (Nematoda) in the Wood Mouse". Journal of Animal Ecology. 59 (1): 363–378. doi:10.2307/5178. JSTOR 5178.
- ↑ al-Bassel, D. A.; Stietieh, F. M.; Farrag, A. M. (2000-04-01). "On the morphology of Heligmosomoides polygyrus (Nematoda-Trichostrongylidae) from the field mouse apodemus sylvaticus". Journal of the Egyptian Society of Parasitology. 30 (1): 43–49. ISSN 1110-0583. PMID 10786017.
- ↑ Pleass, R. J.; Bianco, A. E. (1995-09-01). "The effects of gamma radiation on the development of Heligmosomoides polygyrus bakeri in mice". International Journal for Parasitology. 25 (9): 1099–1109. doi:10.1016/0020-7519(95)00010-Y.
- 1 2 3 4 5 Reynolds, Lisa A.; Filbey, Kara J.; Maizels, Rick M. (2012-10-11). "Immunity to the model intestinal helminth parasite Heligmosomoides polygyrus". Seminars in Immunopathology. 34 (6): 829–846. doi:10.1007/s00281-012-0347-3. ISSN 1863-2297. PMC 3496515
 . PMID 23053394.
. PMID 23053394. - 1 2 Ehrenford, Frank A. (1954-01-01). "The Life Cycle of Nematospiroides dubius Baylis (Nematoda: Heligmosomidae)". The Journal of Parasitology. 40 (4): 480–481. doi:10.2307/3273905. JSTOR 3273905.
- ↑ Bryant, Victoria (1973-09-01). "The Life Cycle of Nematospiroides dubius, Baylis, 1926 (Nematoda: Heligmosomidae)". Journal of Helminthology. 47 (03): 263–268. doi:10.1017/S0022149X00026535. ISSN 1475-2697.
- 1 2 Gregory, Richard D. (1992-01-01). "On the interpretation of host-parasite ecology: Heligmosomoides polygyrus (Nematoda) in wild wood mouse (Apodemus sylvaticus) populations". Journal of Zoology. 226 (1): 109–121. doi:10.1111/j.1469-7998.1992.tb06130.x. ISSN 1469-7998.
- ↑ Grencis, Richard K.; Humphreys, Neil E.; Bancroft, Allison J. (2014-07-01). "Immunity to gastrointestinal nematodes: mechanisms and myths". Immunological Reviews. 260 (1): 183–205. doi:10.1111/imr.12188. ISSN 1600-065X. PMC 4141702
 . PMID 24942690.
. PMID 24942690. - ↑ Filbey, Kara J.; Grainger, John R.; Smith, Katherine A.; Boon, Louis; van Rooijen, Nico; Harcus, Yvonne; Jenkins, Stephen; Hewitson, James P.; Maizels, Rick M. (2014-05-01). "Innate and adaptive type 2 immune cell responses in genetically controlled resistance to intestinal helminth infection". Immunology and Cell Biology. 92 (5): 436–448. doi:10.1038/icb.2013.109. ISSN 0818-9641. PMC 4038150
 . PMID 24492801.
. PMID 24492801. - ↑ Finney, Constance A. M.; Taylor, Matthew D.; Wilson, Mark S.; Maizels, Rick M. (2007-07-01). "Expansion and activation of CD4(+)CD25(+) regulatory T cells in Heligmosomoides polygyrus infection". European Journal of Immunology. 37 (7): 1874–1886. doi:10.1002/eji.200636751. ISSN 0014-2980. PMC 2699425
 . PMID 17563918.
. PMID 17563918. - ↑ Hewitson, James P.; Filbey, Kara J.; Grainger, John R.; Dowle, Adam A.; Pearson, Mark; Murray, Janice; Harcus, Yvonne; Maizels, Rick M. (2011-11-01). "Heligmosomoides polygyrus Elicits a Dominant Nonprotective Antibody Response Directed against Restricted Glycan and Peptide Epitopes". The Journal of Immunology. 187 (9): 4764–4777. doi:10.4049/jimmunol.1004140. ISSN 0022-1767. PMC 4306209
 . PMID 21964031.
. PMID 21964031.