Granadaene
 | |
| Names | |
|---|---|
| IUPAC name
(2S)-5-Amino-2-[[(2E,4E,6E,8E,10E,12E,14E,16E,18E,20E,22E,24E)-27-[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoctacosa-2,4,6,8,10,12,14,16,18,20,22,24-dodecaenoyl]amino]pentanoic acid | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| |
| |
| Properties | |
| C39H52N2O8 | |
| Molar mass | 676.85 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Granadaene is the trivial name of a non-isoprenoid polyene that constitutes the red pigment characteristic of Streptococcus agalactiae (group B streptococcus).
Characteristics
Granadaene is an ornithin-rhamno-dodecaene (molecular mass = 676 Da) with a conjugated system made up of a linear chain of 12 conjugated double bonds.[1][2] Granadaene is dark red, odorless, insoluble in water, methanol, ethanol, diethyl ether, acetone, hexane, dimethyl sulphoxide (DMSO), acetonitrile, tetrahydrofuran, chloroform, and in most solvents, it is soluble in DMSO–0.1% trifluoroacetic acid (TFA).[1] Granadaene, can be extracted from cultures of S.agalactiae in granada broth (granada medium without agar) with 0.1 M potassium hydroxide (KOH) and purified by size-exclusion chromatography on Sephadex LH using DMSO–0.1%TFA.[1]
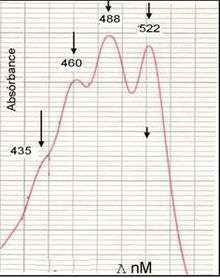
The ultraviolet-visible absorption spectrum of the granadaene (in DMSO/TFA) is almost identical to that of a carotene with a similar conjugated system of double bonds (e.g. alpha-carotene), that is why the GBS pigment was considered to be a carotene for many years.[3]
Biological relevance
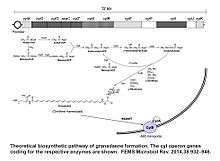
Granadaene is an organic compound produced by S.agalactiae. It is the product of a metabolic pathway similar to that of biosynthesis of fatty acids. The enzymes necessary for the biosynthesis of granadaene in GBS are coded by a gene cluster of 12 genes, the cyl operon, and a pathway for the pigment biosynthesis requiring all the genes of the cyl operon has been proposed.[4][4][5] Like the biosynthesis of the pigment, the hemolytic activity requires also in GBS the 12 genes of the cyl operon.[6][7] The pigment is localized, in GBS, in the cell membrane,[3] where it could play a role in membrane stabilization, similarly to the role of carotenes in other bacterial membranes.[8] It has also been proposed that granadaene is indeed the hemolysin of S.agalactiae, and because the GBS hemolysin is a broad-spectrum cytolysin able to destroy many eukaryotic cells, it is considered an important virulence factor for GBS.[4][5][9]
Production of the red pigment granadaene is a phenotypic trait specific to β-hemolytic GBS, and because of that, detection of red colonies from clinical samples, when cultivated on granada medium, allows the straightforward identification of GBS.[10][11]
In addition to S.agalactiae the presence of granadaene has been reported in Propionibacterium jensenii, where it can cause defects such as red spots in some cheeses.[12] Probably granadaene is also present in other Propionibacterium sp. such as P.thoenii and P.rubrum.[5][12]
References
- 1 2 3 Rosa-Fraile M, Rodríguez-Granger J, Haidour-Benamin A, Cuerva JM, Sampedro A (2006). "Granadaene: Proposed Structure of the Group B Streptococcus Polyenic Pigment" (PDF). Appl Environ Microbiol. 72: 6367–6370. doi:10.1128/aem.00756-06.
- ↑ Paradas M, Jurado R, Haidour A, Rodríguez Granger J, Sampedro Martínez A, de la Rosa Fraile M, Robles R, Justicia J, Cuerva JM (2012). "Clarifying the structure of granadaene: Total synthesis of related analogue - granadaene and confirmation of its absolute stereochemistry". Bioorg Med Chem. 20: 6655–6661. doi:10.1016/j.bmc.2012.09.017.
- 1 2 Merrit K, Jacobs NJ (1978). "Characterization and Incidence of Pigment Production by Human Clinical Group B Streptococci" (PDF). J Clin Microbiol. 8: 105–107. PMC 275130
 . PMID 353069.
. PMID 353069. - 1 2 3 Whidbey C, Harrell MI, Burnside K, Ngo L, Becraft AK, Iyer LM, Aravind L, Hitti J, Waldorf KM, Rajagopal L (2013). "A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta.". J Exp Med. 210: 1265–1281. doi:10.1084/jem.20122753. PMC 3674703
 . PMID 23712433.
. PMID 23712433. - 1 2 3 Rosa-Fraile M, Dramsi S, Spellerberg B (2014). "Group B streptococcal haemolysin and pigment, a tale of twins." (PDF). FEMS Microbiol Rev. 38: 932–946. doi:10.1111/1574-6976.12071.
- ↑ Spellerberg B, Pohl B, Haase G, Martin S, Weber-Heynemann J, Lütticken R (1999). "Identification of genetic determinants for the hemolytic activity of Streptococcus agalactiae by ISS1 transposition." (PDF). J Bacteriol. 181: 3212–3219. PMC 93778
 . PMID 10322024.
. PMID 10322024. - ↑ Spellerberg B, Martin S, Brandt C, Lütticken R (2000). "The cyl genes of Streptococcus agalactiae are involved in the production of pigment". FEMS Microbiol. Lett. 188: 125–128. doi:10.1111/j.1574-6968.2000.tb09182.x.
- ↑ Taylor RF. (1984). "Bacterial triterpenoids.". Microbiol Rev. 48: 181–198. PMC 373008
 . PMID 6387426.
. PMID 6387426. - ↑ Whidbey C, Vornhagen J, Gendrin C, Boldenow E, Samson JM, Doering K, Ngo L, Ezekwe EA Jr, Gundlach JH, Elovitz MA, Liggitt D, Duncan JA, Adams Waldorf KM, Rajagopal L (2015). "A streptococcal lipid toxin induces membrane permeabilization and pyroptosis leading to fetal injury." (PDF). EMBO Mol Med. 7: 488–505. doi:10.15252/emmm.201404883. PMC 4403049
 . PMID 25750210.
. PMID 25750210. - ↑ Rosa-Fraile M, Rodriguez-Granger J, Cueto-Lopez M, Sampedro A, Biel Gaye E, Haro M, Andreu A (1999). "Use of Granada Medium To Detect Group B Streptococcal Colonization in Pregnant Women" (PDF). J Clin Microbiol. 37: 2674–2677.
- ↑ Verani JR, McGee L, Schrag SJ (2010). "Prevention of perinatal group B streptococcal disease: revised guidelines from CDC, 2010" (PDF). MMWR Recomm Rep. 59(RR-10): 1–32.
- 1 2 Vanberg C, Lutnaes BF, Langsrud T, Nees IF, Holo H (2007). "Propionibacterium jensenii produces the polyene pigment granadaene and has hemolytic properties similar to those of Streptococcus agalactiae." (PDF). Appl Environ Microbiol. 75: 5501–5506. doi:10.1128/AEM.00545-07. PMC 2042088
 . PMID 17630313.
. PMID 17630313.