Gould–Jacobs reaction
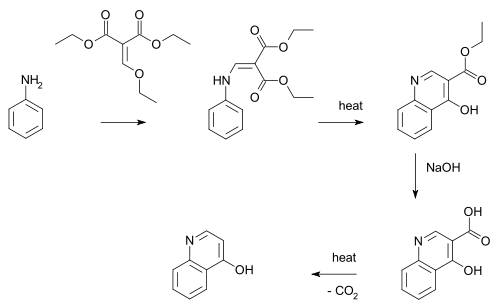
The Gould–Jacobs reaction is an organic synthesis for the preparation of quinolines (more specifically, a 4-Quinolinol).[1] In this reaction aniline or an aniline derivative first reacts with malonic acid derivative ethyl ethoxymethylenemalonate with substitution of the ethoxy group by nitrogen. A benzannulation takes place by application of heat to a quinoline. The ester group is hydrolysed by sodium hydroxide to the carboxylic acid and decarboxylation again by application of heat to 4-hydroxyquinoline.
Examples
An example is the synthesis of 4,7-dichloroquinoline [5]
- Floctafenine and Glafenine are a pair of fenamate NSAIDs whose synthesis rely on the Gould–Jacobs reaction.
- A lot of quinolone antibiotic structures such as Rosoxacin, Oxolinic acid, Droxacin, etc.
References
- ↑ The Synthesis of Certain Substituted Quinolines and 5,6-Benzoquinolines R. Gordon Gould, , Walter Abraham Jacobs J. Am. Chem. Soc.; 1939; 61(10); 2890-2895. doi:10.1021/ja01265a088
- ↑ Li, Jie Jack (2009). "Gould–Jacobs reaction": 263–265. doi:10.1007/978-3-642-01053-8_113.
- ↑ Lengyel, László Csaba; Sipos, Gellért; Sipőcz, Tamás; Vágó, Teréz; Dormán, György; Gerencsér, János; Makara, Gergely; Darvas, Ferenc (2015). "Synthesis of Condensed Heterocycles by the Gould–Jacobs Reaction in a Novel Three-Mode Pyrolysis Reactor". Organic Process Research & Development. 19 (3): 399–409. doi:10.1021/op500354z. ISSN 1083-6160.
- ↑ "Gould-Jacobs Reaction". 2010. doi:10.1002/9780470638859.conrr276.
- ↑ Organic Syntheses, Coll. Vol. 3, p.272 (1955); Vol. 28, p.38 (1948). Link
This article is issued from Wikipedia - version of the 9/21/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.