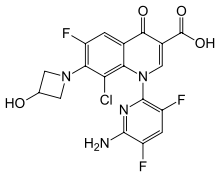
Delafloxacin
 | |
| Clinical data | |
|---|---|
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | 189279-58-1 |
| PubChem (CID) | 487101 |
| ChemSpider | 427049 |
| UNII |
6315412YVF |
| KEGG | D09330 |
| ChEMBL | CHEMBL2105637 |
| Chemical and physical data | |
| Formula | C18H12ClF3N4O4 |
| Molar mass | 440.76 g/mol |
| 3D model (Jmol) | Interactive image |
| |
Delafloxacin (developmental code name RX-3341) is a fluoroquinolone antibiotic being developed by Rib-X Pharmaceuticals, Inc.
It is more active (lower MIC90) than other quinolones against Gram-positive bacteria such as MRSA. In contrast to most approved fluoroquinolones, which are zwitterionic, delafloxacin has an anionic character, which results in a 10-fold increase in delafloxacin accumulation in both bacteria and cells at acidic pH. This property is believed to confer to delafloxacin an advantage for the eradication of Staphylococcus aureus in acidic environments, including intracellular infections.[1]
Clinical trials
Phase II clinical trials have been completed,[2][3][4] including a trial with tigecycline as a comparator[5] The company states delafloxacin met its primary and secondary efficacy endpoints based on the draft guidance from the FDA.
A Phase III trial for acute bacterial skin and skin structure infections (ABSSSI) is due to begin in the 2nd half of 2012.[6]
References
- ↑ Lemaire, S. et al. Contrasting Effects of Acidic pH on the Extracellular and Intracellular Activities of the Anti-Gram-Positive Fluoroquinolones Moxifloxacin and Delafloxacin against Staphylococcus aureus Antimicrob. Agents Chemother. February 2011; 55:649-658 doi:10.1128/AAC.01201-10
- ↑ http://www.bio-medicine.org/biology-technology-1/Rib-X-Pharmaceuticals-Announces-Positive-Phase-2-Study-Results-for-Delafloxacin-and-a--2425-Million-Financing-10093-1/
- ↑ http://clinicaltrials.gov/ct2/show/NCT00719810
- ↑ http://www.medicalnewstoday.com/articles/132200.php "Rib-X Pharmaceuticals Reports Positive Top-Line Results From Phase 2 Study Of Delafloxacin" 9 Dec 2008
- ↑ http://www.citybizlist.com/lstg/lstgDetail.aspx?id=45749 "ABS Ventures Joins $25M Series D Rib-X Pharmaceuticals Inc." 5 Feb 2009
- ↑ Delafloxacin - Pipeline - Rib-X Pharmaceuticals. Accessed: 6/27/2012.