Bcl-2-associated death promoter
| View/Edit Human | View/Edit Mouse |
| Pro-apoptotic Bcl-2 protein, BAD | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 complex of bcl-xl with peptide from bad | |||||||||
| Identifiers | |||||||||
| Symbol | Bcl-2_BAD | ||||||||
| Pfam | PF10514 | ||||||||
| InterPro | IPR018868 | ||||||||
| |||||||||
The Bcl-2-associated death promoter (BAD) protein is a pro-apoptotic member of the Bcl-2 gene family which is involved in initiating apoptosis. BAD is a member of the BH3-only family ,[3] a subfamily of the Bcl-2 family. It does not contain a C-terminal transmembrane domain for outer mitochondrial membrane and nuclear envelope targeting, unlike most other members of the Bcl-2 family.[4] After activation, it is able to form a heterodimer with anti-apoptotic proteins and prevent them from stopping apoptosis.
Mechanism of action
Bax/Bak are believed to initiate apoptosis by forming a pore in the mitochondrial outer membrane that allows cytochrome c to escape into the cytoplasm and activate the pro-apoptotic caspase cascade. The anti-apoptotic Bcl-2 and Bcl-xL proteins inhibit cytochrome c release through the mitochondrial pore and also inhibit activation of the cytoplasmic caspase cascade by cytochrome c.[5]
Dephosphorylated BAD forms a heterodimer with Bcl-2 and Bcl-xL, inactivating them and thus allowing Bax/Bak-triggered apoptosis. When BAD is phosphorylated by Akt/protein kinase B (triggered by PIP3), it forms the BAD-(14-3-3)protein heterodimer. This leaves Bcl-2 free to inhibit Bax-triggered apoptosis.[6] BAD phosphorylation is thus anti-apoptotic, and BAD dephosphorylation (e.g., by Ca2+-stimulated Calcineurin) is pro-apoptotic. The latter may be involved in neural diseases such as schizophrenia.[7]
Interactions
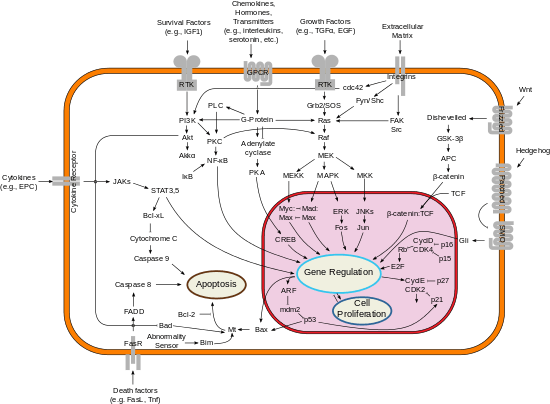
Bcl-2-associated death promoter has been shown to interact with:
See also
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Adachi M, Imai K (2002). "The proapoptotic BH3-only protein BAD transduces cell death signals independently of its interaction with Bcl-2". Cell Death Differ. 9 (11): 1240–7. doi:10.1038/sj.cdd.4401097. PMID 12404123.
- ↑ Hsu SY, Kaipia A, Zhu L, Hsueh AJ (1997). "Interference of BAD (Bcl-xL/Bcl-2-associated death promoter)-induced apoptosis in mammalian cells by 14-3-3 isoforms and P11". Mol. Endocrinol. 11 (12): 1858–67. doi:10.1210/me.11.12.1858. PMID 9369453.
- ↑ Helmreich, E.J.M. (2001) The Biochemistry of Cell Signalling, pp. 238-43
- ↑ E.J.M. (2001) The Biochemistry of Cell Signalling, pp. 242
- ↑ Foster, T.C. et al. (2001) J. Neurosci. 21, 4066-4073, "Calcineurin Links Ca++ Dysregulation with Brain Aging"(
- 1 2 3 4 5 6 Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC (February 2005). "Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function". Mol. Cell. 17 (3): 393–403. doi:10.1016/j.molcel.2004.12.030. PMID 15694340.
- ↑ Jin Z, Xin M, Deng X (April 2005). "Survival function of protein kinase C{iota} as a novel nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-activated bad kinase". J. Biol. Chem. 280 (16): 16045–52. doi:10.1074/jbc.M413488200. PMID 15705582.
- ↑ Strobel T, Tai YT, Korsmeyer S, Cannistra SA (November 1998). "BAD partly reverses paclitaxel resistance in human ovarian cancer cells". Oncogene. 17 (19): 2419–27. doi:10.1038/sj.onc.1202180. PMID 9824152.
- ↑ Zhang H, Nimmer P, Rosenberg SH, Ng SC, Joseph M (August 2002). "Development of a high-throughput fluorescence polarization assay for Bcl-x(L)". Anal. Biochem. 307 (1): 70–5. doi:10.1016/S0003-2697(02)00028-3. PMID 12137781.
- 1 2 Ayllón V, Cayla X, García A, Fleischer A, Rebollo A (July 2002). "The anti-apoptotic molecules Bcl-xL and Bcl-w target protein phosphatase 1alpha to Bad". Eur. J. Immunol. 32 (7): 1847–55. doi:10.1002/1521-4141(200207)32:7<1847::AID-IMMU1847>3.0.CO;2-7. PMID 12115603.
- ↑ Komatsu K, Miyashita T, Hang H, Hopkins KM, Zheng W, Cuddeback S, Yamada M, Lieberman HB, Wang HG (January 2000). "Human homologue of S. pombe Rad9 interacts with BCL-2/BCL-xL and promotes apoptosis". Nat. Cell Biol. 2 (1): 1–6. doi:10.1038/71316. PMID 10620799.
- 1 2 Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ (January 1995). "Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death". Cell. 80 (2): 285–91. doi:10.1016/0092-8674(95)90411-5. PMID 7834748.
- ↑ Petros AM, Nettesheim DG, Wang Y, Olejniczak ET, Meadows RP, Mack J, Swift K, Matayoshi ED, Zhang H, Thompson CB, Fesik SW (Dec 2000). "Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies". Protein Sci. 9 (12): 2528–34. doi:10.1110/ps.9.12.2528. PMC 2144516
 . PMID 11206074.
. PMID 11206074. - ↑ Chattopadhyay A, Chiang CW, Yang E (July 2001). "BAD/BCL-[X(L)] heterodimerization leads to bypass of G0/G1 arrest". Oncogene. 20 (33): 4507–18. doi:10.1038/sj.onc.1204584. PMID 11494146.
- ↑ Iwahashi H, Eguchi Y, Yasuhara N, Hanafusa T, Matsuzawa Y, Tsujimoto Y (November 1997). "Synergistic anti-apoptotic activity between Bcl-2 and SMN implicated in spinal muscular atrophy". Nature. 390 (6658): 413–7. doi:10.1038/37144. PMID 9389483.
- ↑ Komatsu K, Wharton W, Hang H, Wu C, Singh S, Lieberman HB, Pledger WJ, Wang HG (November 2000). "PCNA interacts with hHus1/hRad9 in response to DNA damage and replication inhibition". Oncogene. 19 (46): 5291–7. doi:10.1038/sj.onc.1203901. PMID 11077446.
- 1 2 3 Bae J, Hsu SY, Leo CP, Zell K, Hsueh AJ (October 2001). "Underphosphorylated BAD interacts with diverse antiapoptotic Bcl-2 family proteins to regulate apoptosis". Apoptosis. 6 (5): 319–30. doi:10.1023/A:1011319901057. PMID 11483855.
- ↑ Holmgreen SP, Huang DC, Adams JM, Cory S (June 1999). "Survival activity of Bcl-2 homologs Bcl-w and A1 only partially correlates with their ability to bind pro-apoptotic family members". Cell Death Differ. 6 (6): 525–32. doi:10.1038/sj.cdd.4400519. PMID 10381646.
- 1 2 Hsu SY, Kaipia A, Zhu L, Hsueh AJ (November 1997). "Interference of BAD (Bcl-xL/Bcl-2-associated death promoter)-induced apoptosis in mammalian cells by 14-3-3 isoforms and P11". Mol. Endocrinol. 11 (12): 1858–67. doi:10.1210/me.11.12.1858. PMID 9369453.
- ↑ Yang H, Masters SC, Wang H, Fu H (June 2001). "The proapoptotic protein Bad binds the amphipathic groove of 14-3-3zeta". Biochim. Biophys. Acta. 1547 (2): 313–9. doi:10.1016/S0167-4838(01)00202-3. PMID 11410287.
Further reading
- Tolstrup M, Ostergaard L, Laursen AL, Pedersen SF, Duch M (2004). "HIV/SIV escape from immune surveillance: focus on Nef". Curr. HIV Res. 2 (2): 141–51. doi:10.2174/1570162043484924. PMID 15078178.
- Jiang P, Du W, Wu M (2007). "p53 and Bad: remote strangers become close friends". Cell Res. 17 (4): 283–5. doi:10.1038/cr.2007.19. PMID 17404594.
- Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ (1995). "Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death". Cell. 80 (2): 285–91. doi:10.1016/0092-8674(95)90411-5. PMID 7834748.
- Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ (1996). "Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L)". Cell. 87 (4): 619–28. doi:10.1016/S0092-8674(00)81382-3. PMID 8929531.
- Wang HG, Rapp UR, Reed JC (1996). "Bcl-2 targets the protein kinase Raf-1 to mitochondria". Cell. 87 (4): 629–38. doi:10.1016/S0092-8674(00)81383-5. PMID 8929532.
- Inohara N, Ding L, Chen S, Núñez G (1997). "harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-X(L)". EMBO J. 16 (7): 1686–94. doi:10.1093/emboj/16.7.1686. PMC 1169772
 . PMID 9130713.
. PMID 9130713. - Zha J, Harada H, Osipov K, Jockel J, Waksman G, Korsmeyer SJ (1997). "BH3 domain of BAD is required for heterodimerization with BCL-XL and pro-apoptotic activity". J. Biol. Chem. 272 (39): 24101–4. doi:10.1074/jbc.272.39.24101. PMID 9305851.
- Hsu SY, Kaipia A, Zhu L, Hsueh AJ (1997). "Interference of BAD (Bcl-xL/Bcl-2-associated death promoter)-induced apoptosis in mammalian cells by 14-3-3 isoforms and P11". Mol. Endocrinol. 11 (12): 1858–67. doi:10.1210/me.11.12.1858. PMID 9369453.
- del Peso L, González-García M, Page C, Herrera R, Nuñez G (1997). "Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt". Science. 278 (5338): 687–9. doi:10.1126/science.278.5338.687. PMID 9381178.
- Ottilie S, Diaz JL, Horne W, Chang J, Wang Y, Wilson G, Chang S, Weeks S, Fritz LC, Oltersdorf T (1997). "Dimerization properties of human BAD. Identification of a BH-3 domain and analysis of its binding to mutant BCL-2 and BCL-XL proteins". J. Biol. Chem. 272 (49): 30866–72. doi:10.1074/jbc.272.49.30866. PMID 9388232.
- Huang DC, Adams JM, Cory S (1998). "The conserved N-terminal BH4 domain of Bcl-2 homologues is essential for inhibition of apoptosis and interaction with CED-4". EMBO J. 17 (4): 1029–39. doi:10.1093/emboj/17.4.1029. PMC 1170452
 . PMID 9463381.
. PMID 9463381. - Blume-Jensen P, Janknecht R, Hunter T (1998). "The kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136". Curr. Biol. 8 (13): 779–82. doi:10.1016/S0960-9822(98)70302-1. PMID 9651683.
- Strobel T, Tai YT, Korsmeyer S, Cannistra SA (1998). "BAD partly reverses paclitaxel resistance in human ovarian cancer cells". Oncogene. 17 (19): 2419–27. doi:10.1038/sj.onc.1202180. PMID 9824152.
- Song Q, Kuang Y, Dixit VM, Vincenz C (1999). "Boo, a novel negative regulator of cell death, interacts with Apaf-1". EMBO J. 18 (1): 167–78. doi:10.1093/emboj/18.1.167. PMC 1171112
 . PMID 9878060.
. PMID 9878060. - Yasuda M, Han JW, Dionne CA, Boyd JM, Chinnadurai G (1999). "BNIP3alpha: a human homolog of mitochondrial proapoptotic protein BNIP3". Cancer Res. 59 (3): 533–7. PMID 9973195.
- Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC (1999). "Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD". Science. 284 (5412): 339–43. doi:10.1126/science.284.5412.339. PMID 10195903.
- Holmgreen SP, Huang DC, Adams JM, Cory S (1999). "Survival activity of Bcl-2 homologs Bcl-w and A1 only partially correlates with their ability to bind pro-apoptotic family members". Cell Death Differ. 6 (6): 525–32. doi:10.1038/sj.cdd.4400519. PMID 10381646.
- Ostrerova N, Petrucelli L, Farrer M, Mehta N, Choi P, Hardy J, Wolozin B (1999). "alpha-Synuclein shares physical and functional homology with 14-3-3 proteins". J. Neurosci. 19 (14): 5782–91. PMID 10407019.
- Scheid MP, Schubert KM, Duronio V (1999). "Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase". J. Biol. Chem. 274 (43): 31108–13. doi:10.1074/jbc.274.43.31108. PMID 10521512.
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME (1999). "Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms". Science. 286 (5443): 1358–62. doi:10.1126/science.286.5443.1358. PMID 10558990.
External links
- bcl-Associated Death Protein at the US National Library of Medicine Medical Subject Headings (MeSH)

