Valinomycin
 | |
 | |
| Identifiers | |
|---|---|
| 2001-95-8 | |
| 3D model (Jmol) | [1]: Interactive image |
| ChEBI | CHEBI:28545 |
| ChEMBL | ChEMBL223643 ChEMBL216815 |
| ChemSpider | 21493802 |
| ECHA InfoCard | 100.016.270 |
| |
| Properties | |
| C54H90N6O18 | |
| Molar mass | 1111.32 g/mol |
| Appearance | White solid |
| Melting point | 190 °C (374 °F; 463 K) |
| Solubility | Methanol, ethanol, ethyl acetate, petrol-ether, dichloromethane |
| UV-vis (λmax) | 220 nm |
| Hazards | |
| Main hazards | Neurotoxicant |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
4 mg/kg (oral, rat)[3] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Valinomycin is a naturally occurring dodecadepsipeptide used in the transport of potassium and as an antibiotic. Valinomycin is obtained from the cells of several Streptomyces strains, among which "S. tsusimaensis" and S. fulvissimus.
It is a member of the group of natural neutral ionophores because it does not have a residual charge. It consists of enantiomers D- and L-valine (Val), D-alpha-hydroxyisovaleric acid, and L-lactic acid. Structures are alternately bound via amide and ester bridges. Valinomycin is highly selective for potassium ions over sodium ions within the cell membrane.[4] It functions as a potassium-specific transporter and facilitates the movement of potassium ions through lipid membranes "down" the electrochemical potential gradient.[5] The stability constant K for the potassium-valinomycin complex is nearly 100,000 times larger than that of the sodium-valinomycin complex.[6] This difference is important for maintaining the selectivity of valinomycin for the transport of potassium ions (and not sodium ions) in biological systems.
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[7]
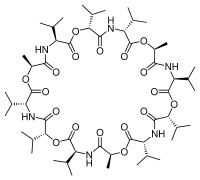
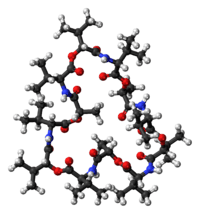
Structure
Valinomycin is a dodecadepsipeptide, that is, it is made of twelve alternating amino acids and esters to form a macrocyclic molecule. The twelve carbonyl groups are essential for the binding of metal ions, and also for solvation in polar solvent. The isopropyl and methyl groups are responsible for solvation in nonpolar solvents. [8] Along with its shape and size this molecular duality is the main reason for its binding properties. K ions must give up their water of hydration to pass through the pore. K+ ions are octahedrally coordinated in a square bipyramidal geometry by 6 carbonyl bonds from Val. In this space of 1.33 Angstrom, Na+ with its 0.95 Angstrom radius, is significantly smaller than the channel, meaning that Na+ cannot form ionic bonds with the amino acids of the pore at equivalent energy as those it gives up with the water molecules. This leads to a 10,000x selectivity for K+ ions over Na+. For polar solvents, valinomycin will mainly expose the carbonyls to the solvent and in nonpolar solvents the isopropyl groups are located predominantly on the exterior of the molecule. This conformation changes when valinomycin is bound to a potassium ion. The molecule is "locked" into a conformation with the isopropyl groups on the exterior. It is not actually locked into configuration because the size of the molecule makes it highly flexible, but the potassium ion gives some degree of coordination to the macromolecule.
Applications
Valinomycin was recently reported to be the most potent agent against severe acute respiratory-syndrome coronavirus (SARS-CoV) in infected Vero E6 cells.
Valinomycin acts as a nonmetallic isoforming agent in potassium selective electrodes.[9][10]
This ionophore is also used in experiments with membrane vesicles to destroy the electrochemical gradient, if such is required by the design of the experiment. [11]
References
- ↑ http://chem.sis.nlm.nih.gov/chemidplus/rn/2001-95-8
- ↑ http://chem.sis.nlm.nih.gov/chemidplus/rn/2001-95-8
- ↑ http://chem.sis.nlm.nih.gov/chemidplus/rn/2001-95-8
- ↑ Lars, Rose; Jenkins ATA (2007). "The effect of the ionophore valinomycin on biomimetic solid supported lipid DPPTE/EPC membranes". Bioelectrochem. 70 (2): 387–393. doi:10.1016/j.bioelechem.2006.05.009. PMID 16875886.
- ↑ Cammann K (1985). "Ion-selective bulk membranes as models". Top. Curr. Chem. 128: 219–258.
- ↑ Rose, M.C.; Henkens, R.W. (1974). "Stability of sodium and potassium complexes of valinomycin". BBA. 372 (2): 426–435. doi:10.1016/0304-4165(74)90204-9.
- ↑ "40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities" (PDF) (July 1, 2008 ed.). Government Printing Office. Retrieved October 29, 2011.
- ↑ Thompson M & Krull UJ (1982). "The electroanalytical response of the bilayer lipid membrane to valinomycin: membrane cholesterol content". Anal. Chim. Acta. 141: 33–47. doi:10.1016/S0003-2670(01)95308-5.
- ↑ Safiulina D, Veksler V, Zharkovsky A, Kaasik A (2006). "Loss of mitochondrial membrane potential is associated with increase in mitochondrial volume: physiological role in neurones". J. Cell. Physiol. 206 (2): 347–353. doi:10.1002/jcp.20476. PMID 16110491.
- ↑ Potassium ionophore Bulletin
- ↑ 1.File.tmp/k_potassium.pdf Potassium ionophore Bulletin]
External links
- Chemical Safety Regulations from New Jersey Department of Health.
- Health information on Scorecard.
- Valinomycin from Fermentek.
- Valinomycin in the Pesticide Properties DataBase (PPDB)