Uveal melanoma
| Uveal melanoma | |
|---|---|
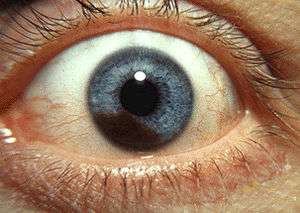 | |
| Iris melanoma | |
| Classification and external resources | |
| Specialty | Oncology |
| ICD-10 | C69 |
| ICD-9-CM | 190 |
| ICD-O | M8720/3 |
| OMIM | 155720 |
| DiseasesDB | 2614 |
| MedlinePlus | 001022 |
| eMedicine | oph/403 |
| MeSH | D014604 |
Uveal melanoma is a cancer (melanoma) of the eye involving the iris, ciliary body, or choroid (collectively referred to as the uvea). Tumors arise from the pigment cells (melanocytes) that reside within the uvea giving color to the eye. These melanocytes are distinct from the retinal pigment epithelium cells underlying the retina that do not form melanomas.
Epidemiology
Uveal melanomas are the most common primary intraocular tumor in adults.[1] The incidence has remained stable for several years.
Cause
The cause of uveal melanoma is unclear, but UV light is a risk factor. Uveal nevi are common (10% of caucasians), but rarely progress to melanoma.
Types
Uveal melanomas, often referred to by the media and in the general population as ocular melanomas, may arise from any of the three parts of the uvea, and are sometimes referred to by their location, as choroidal melanoma, ciliary body melanoma, or iris melanoma. Large tumors often encompass multiple parts of the uvea and can be named accordingly. True iris melanomas, originating from within the iris as opposed to originating elsewhere and invading the iris, are distinct in their etiology and prognosis, such that the other tumors are often referred to collectively as Posterior uveal melanomas.
Iris melanoma
Uveal tumors can originate from melanocytes residing within the iris. Benign melanocytic tumors, such as iris freckles and moles (nevi), are common and pose no health risks, unless they show signs of malignancy, in which case they are classified as iris melanomas. Though derived from uveal melanocytes, iris melanomas share more in common with cutaneous (skin) melanomas, in that they frequently harbor BRAF mutations associated with ultraviolet damage.[2][3] Iris melanomas are much less likely to metastasize than other uveal melanomas, and less likely to impair vision if detected and treated early. Approximately 5% of uveal melanomas involve the iris.[4]
Posterior uveal melanoma

Benign melanocytic tumors of the choroid, such as choroidal freckles and nevi, are very common and pose no health risks, unless they show signs of malignancy, in which case they are considered melanomas.[5][6] Uveal melanoma is distinct from most skin melanomas associated with ultraviolet exposure; however, it shares several similarities with non-sun-exposed melanomas, such as acral melanomas and mucosal melanomas. BRAF mutations are extremely rare in posterior uveal melanomas;[7] instead, uveal melanomas frequently harbor GNAQ/GNA11 mutations, a trait shared with blue nevi, Nevus of Ota, and Ocular melanosis.[8][9] As seen in BRAF, mutations in GNAQ/GNA11 are early events in tumorigenesis and are not prognostic for tumor stage or later metastatic spread.[10] In contrast, mutations in the gene BAP1 are strongly linked to metastatic spread and patient survival.[11] Incidence of posterior uveal melanoma is highest among people with light skin and blue eyes. Other risk factors, such as blue light exposure and arc welding have been put forward, but are still debated in the field. Mobile phone use is not a risk factor for uveal melanoma.[12]
Treatment
The treatment protocol for uveal melanoma has been directed by many clinical studies, the most important being "The Collaborative Ocular Melanoma Study" (COMS). The treatment varies depending upon many factors, chief among them, the size of the tumor. Primary treatment can involve removal of the affected eye (enucleation); however, this is now reserved for cases of extreme tumor burden or other secondary problems. Advances in radiation therapies have significantly decreased the number of patients treated by enucleation in developed countries. The most common radiation treatment is plaque brachytherapy, in which a small disc-shaped shield (plaque) encasing radioactive seeds (most often Iodine-125, though Ruthenium-106 and Palladium-103 are also used) is attached to the outside surface of the eye, overlying the tumor. The plaque is left in place for a few days and then removed. The risk of metastasis after plaque radiotherapy is the same as that of enucleation, suggesting that micrometastatic spread occurs prior to treatment of the primary tumor. Other modalities of treatment include transpupillary thermotherapy, external beam proton therapy, resection of the tumor, Gamma Knife stereotactic radiosurgery or a combination of different modalities. Different surgical resection techniques can include trans-scleral partial choroidectomy, and transretinal endoresection.
Prognostic factors
Several clinical and pathological prognostic factors have been identified that are associated with higher risk of metastasis of uveal melanomas. These include large tumor size, ciliary body involvement, presence of orange pigment overlying the tumor, and older patient age.[13][14] Likewise several histological and cytological factors are associated with higher risk of metastasis including presence and extent of cells with epithelioid morphology, presence of looping extracellular matrix patterns, increased infiltration of immune cells,[1] as well as staining with several immunohistochemical markers.[15]
The most important genetic alteration associated with poor prognosis in uveal melanoma is inactivation of BAP1, which most often occurs through mutation of one allele and subsequent loss of an entire copy of Chromosome 3 (Monosomy 3) to unmask the mutant copy.[11] Because of this function in inactivation of BAP1, monosomy 3 correlates strongly with metastatic spread[16] Where BAP1 mutation status is not available, gains on chromosomes 6 and 8 can be used to refine the predictive value of the Monosomy 3 screen, with gain of 6p indicating a better prognosis and gain of 8q indicating a worse prognosis in disomy 3 tumors.[17] In rare instances, monosomy 3 tumors may duplicate the BAP1-mutant copy of the chromosome to return to a disomic state referred to as isodisomy.[18] Thus, isodisomy 3 is prognostically equivalent to monosomy 3, and both can be detected by tests for chromosome 3 loss of heterozygosity.[19] Monosomy 3, along with other chromosomal gains, losses, amplifications, and LOH, can be detected in fresh or paraffin embedded samples by virtual karyotyping.
The most accurate prognostic factor is molecular classification by gene expression profiling of uveal melanomas. This analysis has been used to identify two subclasses of uveal melanomas: class 1 tumors that have a very low risk of metastasis and class 2 tumors that have a very high risk of metastasis.[20][21] Gene expression profiling outperforms all of the above-mentioned factors at predicting metastatic spread of the primary tumor, including monosomy 3.[22][23][24]
Metastasis
Because there are no lymphatic channels to the uveal tract, metastasis occurs through local extension and/or blood borne dissemination.[25] The most common site of metastasis for uveal melanoma is the liver;[1] the liver is the first site of metastasis for 80%-90% of ocular melanoma patients.[26] Other common sites of metastasis include the lung, bones and just beneath the skin (subcutaneous). Approximately 50 percent of patients will develop metastases within 15 years after treatment of the primary tumor, and the liver will be involved 90% of the time.[27] Metastasis can occur more than 10 years after treatment of the primary tumor, and patients should not be considered cured even after a 10-year interval of monitoring.[28] Molecular features of the tumor including Chromosome 3 status, Chromosome 6p status, and Chromosome 8q status and gene expression profiling (such as the DecisionDx-UM test) can be used to adjust this likelihood of metastasis for an individual patient.
The average survival time after diagnosis of liver metastases depends on the extent of systemic spread. The disease-free interval, the performance status, the liver substitution by metastases and the serum level of lactic dehydrogenase are the most important prognostic factors for metastatic uveal melanoma.[29] There is currently no cure for metastatic uveal melanoma.
Surveillance
Currently, there is no consensus regarding type or frequency of scans following diagnosis and treatment of the primary eye tumor. Of the 50% of patients who develop metastatic disease, more than 90% of patients will develop liver metastases. As such, the majority of surveillance techniques are focused on the liver. These include abdominal magnetic resonance imaging (MRI), abdominal ultrasound and liver function tests. The scientific community is currently working to develop guidelines, but until then, each patient must take into consideration their individual clinical situation and discuss appropriate surveillance with their doctors.[30] Some ophthalmologists have also found promise with the use of intravitreal avastin injections in patients suffering from radiation-induced retinopathy, a side effect of plaque brachytherapy treatment, as well as imaging surveillance with SD-OCT.
See also
References
- 1 2 3 Kumar, Vinay (2009). "Uvea: Neoplasms". Robbins and Cotran Pathologic Basis of Disease, Professional Edition. (8th ed.). Philadelphia, PA: Elsevier. ISBN 978-1-4377-0792-2.
- ↑ Henriquez F, Janssen C, Kemp EG, Roberts F (2007). "The T1799A BRAF mutation is present in iris melanoma". Invest Ophthalmol Vis Sci. 48 (11): 4897–4900. doi:10.1167/iovs.07-0440. PMID 17962436.
- ↑ Hocker T, Tsao H (2007). "Ultraviolet radiation and melanoma: a systematic review and analysis of reported sequence variants". Hum Mutat. 28 (6): 578–588. doi:10.1002/humu.20481. PMID 17295241.
- ↑ Damato B; Coupland S (2008). "Ocular melanoma". Melanoma Molecular Map Project. Retrieved 2013-02-02.
- ↑ Augsburger JJ (1993). "Is observation really appropriate for small choroidal melanomas". Trans Am Ophthalmol Soc. 91: 147–175. PMC 1298464
 . PMID 8140689.
. PMID 8140689. - ↑ Shields CL, Demirci H, Materin MA, Marr BP, Mashayekhi A, Shields JA (2004). "Clinical factors in the identification of small choroidal melanoma". Can J Ophthalmol. 39 (4): 351–357. PMID 15327099.
- ↑ Malaponte G, Libra M, Gangemi P, Bevelacqua V, Mangano K, D'Amico F, Mazzarino MC, Stivala F, McCubrey JA, Travali S (2006). "Detection of BRAF gene mutation in primary choroidal melanoma tissue". Cancer Biol Ther. 5 (2): 225–227. doi:10.4161/cbt.5.2.2429. PMID 16410717.
- ↑ Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O'Brien JM, Simpson EM, Barsh GS, Bastian BC (2009). "Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi". Nature. 457 (7229): 599–602. doi:10.1038/nature07586. PMC 2696133
 . PMID 19078957.
. PMID 19078957. - ↑ Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, Obenauf AC, Wackernagel W, Green G, Bouvier N, Sozen MM, Baimukanova G, Roy R, Heguy A, Dolgalev I, Khanin R, Busam K, Speicher MR, O'Brien J, Bastian BC (2010). "Mutations in GNA11 in uveal melanoma". N Engl J Med. 363 (23): 2191–2199. doi:10.1056/NEJMoa1000584. PMC 3107972
 . PMID 21083380.
. PMID 21083380. - ↑ Onken MD, Worley LA, Long MD, Duan S, Council ML, Bowcock AM, Harbour JW (2008). "Oncogenic mutations in GNAQ occur early in uveal melanoma". Invest Ophthalmol Vis Sci. 49 (12): 5230–5234. doi:10.1167/iovs.08-2145. PMC 2634606
 . PMID 18719078.
. PMID 18719078. - 1 2 Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, Council ML, Matatall KA, Helms C, Bowcock AM (2010). "Frequent mutation of BAP1 in metastasizing uveal melanomas". Science. 330 (6009): 1410–1413. doi:10.1126/science.1194472. PMC 3087380
 . PMID 21051595.
. PMID 21051595. - ↑ Stang A, Schmidt-Pokrzywniak A, Lash TL, Lommatzsch PK, Taubert G, Bornfeld N, Jöckel KH (2009). "Mobile phone use and risk of uveal melanoma: results of the risk factors for uveal melanoma case-control study". J Natl Cancer Inst. 101 (2): 120–123. doi:10.1093/jnci/djn441. PMC 2639317
 . PMID 19141780.
. PMID 19141780. - ↑ Augsburger JJ, Gamel JW (1990). "Clinical prognostic factors in patients with posterior uveal malignant melanoma". Cancer. 66 (7): 1596–1600. doi:10.1002/1097-0142(19901001)66:7<1596::AID-CNCR2820660726>3.0.CO;2-6. PMID 2208011.
- ↑ "General Information About Intraocular (Uveal) Melanoma". National Institutes of Health. Retrieved 26 November 2013.
- ↑ Pardo M, Dwek RA, Zitzmann N (2007). "Proteomics in uveal melanoma research: opportunities and challenges in biomarker discovery". Expert Rev Proteomics. 4 (2): 273–286. doi:10.1586/14789450.4.2.273. PMID 17425462.
- ↑ Prescher G, Bornfeld N, Hirche H, Horsthemke B, Jöckel KH, Becher R (1996). "Prognostic implications of monosomy 3 in uveal melanoma". Lancet. 347 (9010): 1222–1225. doi:10.1016/S0140-6736(96)90736-9. PMID 8622452.
- ↑ Damato BE, Dopierala J, Klaasen A, van Dijk M, Sibbring J, Coupland S (2009). "Multiplex Ligation-Dependent Probe Amplification of Uveal Melanoma: Correlation with Metastatic Death". Invest Ophthalmol Vis Sci. 50 (7): 3048–55. doi:10.1167/iovs.08-3165. PMID 19182252.
- ↑ White VA, McNeil BK, Horsman DE (1998). "Acquired homozygosity (isodisomy) of chromosome 3 in uveal melanoma". Cancer Genet Cytogenet. 102 (1): 40–45. doi:10.1016/S0165-4608(97)00290-2. PMID 9530338.
- ↑ Onken MD, Worley LA, Person E, Char DH, Bowcock AM, Harbour JW (2007). "Loss of heterozygosity of chromosome 3 detected with single nucleotide polymorphisms is superior to monosomy 3 for predicting metastasis in uveal melanoma". Clin Cancer Res. 13 (10): 2923–2937. doi:10.1158/1078-0432.CCR-06-2383. PMID 17504992.
- ↑ Tschentscher F, Hüsing J, Hölter T, Kruse E, Dresen IG, Jöckel KH, Anastassiou G, Schilling H, Bornfeld N, Horsthemke B, Lohmann DR, Zeschnigk M (2003). "Tumor classification based on gene expression profiling shows that uveal melanomas with and without monosomy 3 represent two distinct entities". Cancer Res. 63 (10): 2578–2584. PMID 12750282.
- ↑ Onken MD, Worley LA, Ehlers JP, Harbour JW (2004). "Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death". Cancer Res. 64 (20): 7205–7209. doi:10.1158/0008-5472.CAN-04-1750. PMID 15492234.
- ↑ Petrausch U, Martus P, Tönnies H, Bechrakis NE, Lenze D, Wansel S, Hummel M, Bornfeld N, Thiel E, Foerster MH, Keilholz U (2008). "Significance of gene expression analysis in uveal melanoma in comparison to standard risk factors for risk assessment of subsequent metastases". Eye. 22 (8): 997–1007. doi:10.1038/sj.eye.6702779. PMID 17384575.
- ↑ van Gils W, Lodder EM, Mensink HW, Kiliç E, Naus NC, Brüggenwirth HT, van Ijcken W, Paridaens D, Luyten GP, de Klein A (2008). "Gene expression profiling in uveal melanoma: two regions on 3p related to prognosis". Invest Ophthalmol Vis Sci. 49 (10): 4254–4262. doi:10.1167/iovs.08-2033. PMID 18552379.
- ↑ Worley LA, Onken MD, Person E, Robirds D, Branson J, Char DH, Perry A, Harbour JW (2007). "Transcriptomic versus chromosomal prognostic markers and clinical outcome in uveal melanoma". Clin Cancer Res. 13 (5): 1466–1471. doi:10.1158/1078-0432.CCR-06-2401. PMID 17332290.
- ↑ "Classification and Stage Information for Intraocular (Uveal) Melanoma". National Cancer Institute. Retrieved 2013-07-04.
- ↑ "Prognostic Indicators". Ocular Melanoma Foundation. 2012. Retrieved 2013-02-02.
- ↑ Spagnolo, Francesco; Graziano Caltabiano; Paola Queirolo (January 2012). "Uveal melanoma". Cancer Treatment Reviews. 38 (5): 549–553. doi:10.1016/j.ctrv.2012.01.002. Retrieved 24 November 2013.
- ↑ Kolandjian, NA; Wei C; Patel SP; Richard JL; Dett T; Papadopoulos NE; Bedikian AY (October 2013). "Delayed systemic recurrence of uveal melanoma". American Journal of Clinical Oncology. 36 (5): 443–9. doi:10.1097/COC.0b013e3182546a6b. PMID 22706174.
- ↑ Valpione Sara; et al. (Mar 2015). "Development and external validation of a prognostic nomogram for metastatic uveal melanoma.". PLOS ONE. 10 (3): e0120181. doi:10.1371/journal.pone.0120181. PMC 4363319
 . PMID 25780931.
. PMID 25780931. - ↑ "MRF CURE OM". Melanoma Research Foundation. Retrieved 30 March 2012.
Foundations supporting uveal melanoma research, education, and advocacy
- CURE Ocular Melanoma (an initiative of the Melanoma Research Foundation)
- The Eye Cancer Foundation
- OcuMel UK - Charity supporting people affected by Ocular Melanoma in the UK
- The Tumori Foundation
- Ocular Melanoma Foundation
- A Cure in Sight ( non profit supporting OM patients and ongoing research )
External links
- Ocular Oncology Service (Dr. Shields), Wills Eye Hospital, Philadelphia, PA
- MyUVEALMelanoma - Overview of DecisionDX-UM from Castle BioSciences
- The Ocular Oncology Service at Washington University St. Louis
- The Eye Cancer Network
- Ocular Oncology - Bascom Palmer Eye Institute
- All NCI-approved clinical trials for uveal melanoma that has spread to the liver
- National Cancer Institute Clinical Trials Search Results
- EyeMelanoma.org
- Uveal Melanoma MR Scans of ocular melanoma
- The New York Eye Cancer Center