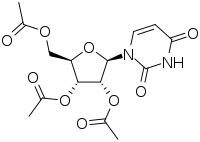
Uridine triacetate
 | |
| Clinical data | |
|---|---|
| Trade names | Vistogard, Xuriden |
| Routes of administration | Oral granules |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Pyrimidine catabolic pathway |
| Onset of action | Tmax = 2-3 hours |
| Biological half-life | 2-2.5 hours |
| Excretion | Renal |
| Identifiers | |
| DrugBank | DB09144 |
| ECHA InfoCard | 100.021.710 |
| Chemical and physical data | |
| Formula | C15H18Cl0N2O9S0 |
| Molar mass | 370.31 g·mol−1 |
Uridine triacetate is a drug used in the treatment of hereditary orotic aciduria[1] and to treat patients following an overdose of chemotherapy drugs 5-fluorouracil or capecitabine, or in patients exhibiting early-onset, severe or life-threatening toxicity affecting the cardiac or central nervous system, and/or early-onset, unusually severe adverse reactions (e.g., gastrointestinal toxicity and/or neutropenia) within 96 hours following the end of 5-fluorouracil or capecitabine administration.[2][3]
Uridine triacetate was developed, manufactured and distributed by Wellstat Therapeutics and it is marketed in USA by BTG. Also, It was granted breakthrough therapy designation by FDA in 2015.
Uridine triacetate is a prodrug of uridine.[4]
References
- ↑ HIGHLIGHTS OF PRESCRIBING INFORMATION OF XURIDEN
- ↑ BTG Announces FDA Approval of VISTOGARD® (Uridine Triacetate) as Antidote to Overdose and Early Onset, Severe, or Life-Threatening Toxicities from Chemotherapy Drugs 5-Fluorouracil (5-FU) or Capecitabine
- ↑ "FDA Approved Drugs:Uridine Triacetate". FDA. 2015-12-11. Retrieved 2016-04-29.
- ↑ "Uridine triacetate". DrugBank.
This article is issued from Wikipedia - version of the 7/22/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.