Ullmann reaction
| Ullmann reaction | |
|---|---|
| Named after | Fritz Ullmann |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | ullmann-reaction |
| RSC ontology ID | RXNO:0000040 |
The Ullmann reaction or Ullmann coupling [1] is a coupling reaction between aryl halides and copper. The reaction is named after Fritz Ullmann.[2]

A typical example is the coupling of 2 o-chloronitrobenzene reactants to form 2,2'-dinitrobiphenyl with a copper - bronze alloy.[3]

The traditional version of the Ullmann reaction requires harsh reaction conditions, and the reaction has a reputation for erratic yields. Since its discovery some improvements and alternative procedures have been introduced.[4]
The reaction mechanism of the Ullmann reaction is extensively studied. Electron spin resonance rules out a radical intermediate. The oxidative addition / reductive elimination sequence observed with palladium catalysts is unlikely for copper because copper(III) is rarely observed. The reaction probably involves the formation of an organocopper compound (RCuX) which reacts with the other aryl reactant in a nucleophilic aromatic substitution. Alternative mechanisms do exist such as σ-bond metathesis.[5]
The classical Ullmann reaction is limited to electron deficient aryl halides and requires harsh reaction conditions. Modern variants of the Ullman reaction employing palladium and nickel have widened the substrate scope of the reaction and rendered reaction conditions more mild. Yields are generally still moderate, however.[6] In organic synthesis this reaction is often replaced by palladium coupling reactions such as the Heck reaction, the Hiyama coupling and the Sonogashira coupling.
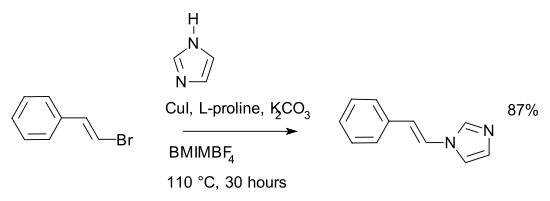
In a variation of the Ullmann reaction, (2-bromovinyl)-benzene is reacted with imidazole in an ionic liquid, BMIMBF4*, to give N-(2-phenylvinyl)-imidazole.[7] The reaction requires (L)-proline catalysis.
Note
- * BMIMBF4 stands for the ionic liquid, 1-butyl-3-methylimidazolium tetrafluoroborate
See also
- Copper(I)-thiophene-2-carboxylate, a copper reagent used in the Ullmann reaction
- Wurtz-Fittig reaction, a similar reaction useful for alkylbenzenes synthesis
References
- ↑ P.E. Fanta (1974). "The Ullmann Synthesis of Biaryls". Synthesis. 1974: 9–21. doi:10.1055/s-1974-23219.
- ↑ F. Ullmann; Jean Bielecki (1901). "Ueber Synthesen in der Biphenylreihe". Chemische Berichte. 34 (2): 2174–2185. doi:10.1002/cber.190103402141.
- ↑ Reynold C. Fuson and E. A. Cleveland, "2,2'-dinitrobiphenyl", Organic Syntheses, Coll. Vol. 3, 339. Online article
- ↑ J. Hassan; M. Sevignon; C. Gozzi; E. Schulz; M. Lemaire (2002). "Aryl-Aryl Bond Formation One Century after the Discovery of the Ullmann Reaction". Chemical Reviews. 102 (5): 1359–1470. doi:10.1021/cr000664r. PMID 11996540.
- ↑ Derek van Allen, PhD Thesis, University of Massachusetts Amherst 2004. Electronic thesis
- ↑ Nelson, T. D.; Crouch, R. D. Org. React. 2004, 63, 265. doi:10.1002/0471264180.or063.03
- ↑ Zhiming Wang, Weiliang Bao and Yong Jiang, "L-Proline promoted Ullmann-type reaction of vinyl bromides with imidazoles in ionic liquids", Chemical Communications, 2005, 2849-51 Abstract