Tumor-infiltrating lymphocytes
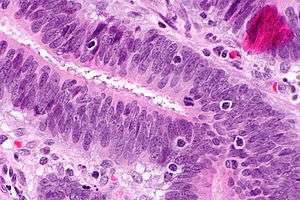
Tumor-infiltrating lymphocytes, also tumour infiltrating lymphocytes, are white blood cells that have left the bloodstream and migrated into a tumor. They are mononuclear immune cells, a mix of different types of cells (i.e., T cells, B cells, NK cells, macrophages) in variable proportions, T cells being the most abundant cells.[1]
They can often be found in the stroma and within the tumour itself.[1]
TILs are implicated in killing tumor cells. The presence of lymphocytes in tumors is often associated with better clinical outcomes.[2][3][4]
Detection and characteristics
When TILs are present the lymphocytes are found between the tumor cells; cells in the stroma surrounding the tumor cells do not count.[5] It should be noted that histologic definitions for TILs vary.
CD3 has been used to detect lymphocytes in tumor samples.[4]
Associations with cancer prognosis
Colorectal cancer
In colorectal cancer, they are associated with microsatellite instability cancers, as may be seen in Lynch syndrome.[6]
TILs are needed for checkpoint inhibitor therapy to work in GI cancers.[4]
Melanoma
They are an important prognostic factor in melanoma and higher levels being associated with a better outcome.[7][8]
Use in an autologous cell therapy
They are key to an experimental autologous cell therapy (Contego) for metastatic melanoma.[9]
Use as an adoptive cell transfer therapy
History
The use of TILs as an adoptive cell transfer therapy to treat cancer was pioneered by Dr. Steven Rosenberg at the National Cancer Institute. Autologous lymphocytes are isolated from patients’ tumors and cultured to large numbers of cells in vitro. Prior to TIL treatment, patients are given nonmyeloablative chemotherapy to deplete native lymphocytes that can inhibit the response. Once lymphodepletion is completed, patients are infused with TILs in combination with interleukin 2 (IL-2). Lion Biotechnologies is developing adoptive cell transfer with TILs as a cancer therapy.
for Melanoma
Several centres are currently working on TIL melanoma treatment protocol, including the Ella Institute in Sheba Hospital, Israel[10] and Copenhagen University Hospital at Herlev, Denmark [11][12]
Clinical trials have used TILs to treat patients with metastatic melanoma. Tumor reduction of 50% or more was observed in about half of patients.[13][14][15][16] Some patients experienced complete responses with no detectable tumor remaining years after treatment.[17]
for Other cancers
Clinical trials using TILs to treat digestive tract cancers, such as colorectal cancer,[18] and cancers associated with the human papilloma virus (HPV), such as cervical cancer,[19] are ongoing.
Under investigation are the use of TILs to treat other tumors, including lung, ovarian, bladder, and breast.
See also
References
- 1 2 Breast Cancer Immunology. Oncology Times: 10 May 2016 - Volume 38 - Issue 9 - p 18–19 doi: 10.1097/01.COT.0000483221.52404.e3
- ↑ Vánky F, Klein E, Willems J, et al. (1986). "Lysis of autologous tumor cells by blood lymphocytes tested at the time of surgery. Correlation with the postsurgical clinical course". Cancer Immunology, Immunotherapy. 21 (1): 69–76. doi:10.1007/BF00199380. PMID 3455878.
- ↑ Zhang L, Conejo-Garcia JR, Katsaros D, et al. (January 2003). "Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer". The New England Journal of Medicine. 348 (3): 203–13. doi:10.1056/NEJMoa020177. PMID 12529460.
- 1 2 3 Immunotherapy Doubts Fading in GI Cancers. April 2016
- ↑ Garg, K.; Soslow, RA. (Aug 2009). "Lynch syndrome (hereditary non-polyposis colorectal cancer) and endometrial carcinoma.". J Clin Pathol. 62 (8): 679–84. doi:10.1136/jcp.2009.064949. PMID 19638537.
- ↑ Iacopetta, B.; Grieu, F.; Amanuel, B. (Dec 2010). "Microsatellite instability in colorectal cancer.". Asia Pac J Clin Oncol. 6 (4): 260–9. doi:10.1111/j.1743-7563.2010.01335.x. PMID 21114775.
- ↑ Spatz; et al. (2007). "Protective effect of a brisk tumor infiltrating lymphocyte infiltrate in melanoma: An EORTC melanoma group study.". Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (June 20 Supplement), 2007: 8519.
- ↑ Galon; et al. (2006). "Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome.". Science 29 September 2006: 313 (5795), 1960-1964.
- ↑ "Genesis Biopharma expands clinical focus to develop Contego for Stage IV metastatic melanoma". June 2011.
- ↑ "Clinical Responses in a Phase II Study Using Adoptive Transfer of Short-term Cultured Tumor Infiltration Lymphocytes in Metastatic Melanoma Patients." (PDF).
- ↑ "Adoptive cell therapy with autologous tumor infiltrating lymphocytes and low-dose Interleukin-2 in metastatic melanoma patients.".
- ↑ "Methods to Improve Adoptive T-Cell Therapy for Melanoma: IFN-γ Enhances Anticancer Responses of Cell Products for Infusion".
- ↑ Dudley ME, Yang JC, Sherry R, et al. (November 2008). "Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens". Journal of Clinical Oncology. 26 (32): 5233–9. doi:10.1200/JCO.2008.16.5449. PMC 2652090
 . PMID 18809613.
. PMID 18809613. - ↑ Radvanyi LG, Bernatchez C, Zhang M, et al. (December 2012). "Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients". Clinical Cancer Research. 18 (24): 6758–70. doi:10.1158/1078-0432.CCR-12-1177. PMC 3525747
 . PMID 23032743.
. PMID 23032743. - ↑ Pilon-Thomas S, Kuhn L, Ellwanger S, et al. (October 2012). "Efficacy of adoptive cell transfer of tumor-infiltrating lymphocytes after lymphopenia induction for metastatic melanoma". Journal of Immunotherapy. 35 (8): 615–20. doi:10.1097/CJI.0b013e31826e8f5f. PMID 22996367.
- ↑ Besser MJ, Shapira-Frommer R, Treves AJ, et al. (May 2010). "Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients". Clinical Cancer Research. 16 (9): 2646–55. doi:10.1158/1078-0432.CCR-10-0041. PMID 20406835.
- ↑ Rosenberg SA, Yang JC, Sherry RM, et al. (July 2011). "Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy". Clinical Cancer Research. 17 (13): 4550–7. doi:10.1158/1078-0432.CCR-11-0116. PMC 3131487
 . PMID 21498393.
. PMID 21498393. - ↑ Clinical trial number NCT01174121 for "A Phase II Study Using Short-Term Cultured, CD8+-Enriched Autologous Tumor-infiltrating Lymphocytes Following a Lymphocyte Depleting Regimen in Metastatic Digestive Tract Cancers" at ClinicalTrials.gov
- ↑ Clinical trial number NCT01585428 for "A Phase II Study of Lymphodepletion Followed by Autologous Tumor-Infiltrating Lymphocytes and High-Dose Adesleukin for Human Papillomavirus-Associated Cancers" at ClinicalTrials.gov
External links
- Tumor infiltrating lymphocyte entry in the public domain NCI Dictionary of Cancer Terms
- Lion Biotechnologies, Inc.