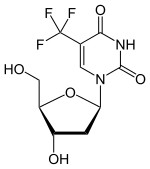
Trifluridine
 | |
| Clinical data | |
|---|---|
| Trade names | Viroptic; Lonsurf (+tipiracil) |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Eye drops; tablets (+tipiracil) |
| ATC code | S01AD02 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability |
Negligible (eye drops); ≥57% (oral) |
| Protein binding | >96% |
| Metabolism | Thymidine phosphorylase |
| Biological half-life |
12 minutes (eye drops); 1.4–2.1 hrs (combination with tipiracil) |
| Excretion | Mostly via urine |
| Identifiers | |
| |
| Synonyms | α,α,α-trifluorothymidine; 5-trifluromethyl-2′-deoxyuridine; FTD5-trifluoro-2′-deoxythymidine; TFT; CF3dUrd; FTD; F3TDR; F3Thd |
| CAS Number |
70-00-8 |
| PubChem (CID) | 6256 |
| DrugBank |
DB00432 |
| ChemSpider |
6020 |
| UNII |
RMW9V5RW38 |
| KEGG |
D00391 |
| ChEBI |
CHEBI:75179 |
| ChEMBL |
CHEMBL1129 |
| ECHA InfoCard | 100.000.657 |
| Chemical and physical data | |
| Formula | C10H11F3N2O5 |
| Molar mass | 296.2 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Trifluridine (also called trifluorothymidine or TFT) is an anti-herpesvirus antiviral drug, used primarily on the eye. It was sold under the trade name Viroptic by Glaxo Wellcome, now merged into GlaxoSmithKline. The brand is now owned by Monarch Pharmaceuticals, which is wholly owned by King Pharmaceuticals.
Trifluridine was approved for medical use in 1980.[1] It is also a component of the anti-cancer drug trifluridine/tipiracil, which is taken by mouth.
Medical uses
Trifluridine eye drops are used for the treatment of keratitis and keratoconjunctivitis caused by the herpes simplex virus types 1 and 2, as well as for prevention and treatment of vaccinia virus infections of the eye.[2]
A Cochrane Systematic Review showed that trifluridine was a more effective treatment than idoxuridine or vidarabine, significantly increasing the relative number of successfully healed eyes in 14 days.[3]
For cancer treatment, the combination trifluridine/tipiracil is used.
Adverse effects
Common side effects of trifluridine eye drops include transient burning, stinging, local irritation, and edema of the eyelids.[2]
Adverse effects of the anti-cancer formulation have only been evaluated for the combination trifluridine/tipiracil, not for the individual components.
Interactions
Only in vitro interaction studies are available. In these, trifluridine used the concentrative nucleoside transporter 1 (CNT1) and equilibrative nucleoside transporters 1 (ENT1) and 2 (ENT2). Drugs that interact with these transporters could influence blood plasma concentrations of trifluridine. Being a thymidine phosphorylase inhibitor, trifluridine could also interact with substrates of this enzyme such as zidovudine.[4]
For the eye drops, trifluridine absorption is negligible,[2] rendering interactions basically irrelevant.
Pharmacology
Mechanism of action (eye drops)
It is a nucleoside analogue, a modified form of deoxyuridine, similar enough to be incorporated into viral DNA replication, but the –CF3 group added to the uracil component blocks base pairing, thus interfering with viral DNA replication.
Pharmacokinetics (eye drops)
Trifluridine passes the cornea and is found in the aqueous humour. Systemic absorption is negligible.[2]
Pharmacokinetics (oral)

Pharmacokinetic data of oral trifluridine have only been evaluated in combination with tipiracil, which significantly affects biotransformation of the former. At least 57% of trifluridine are absorbed from the gut, and highest blood plasma concentrations are reached after two hours in cancer patients. The substance has no tendency to accumulate in the body. Plasma protein binding is over 96%. Trifluridine is metabolised by the enzyme thymidine phosphorylase to 5-trifluoromethyl-2,4(1H,3H)-pyrimidinedione (FTY), and also by glucuronidation. Elimination half-life is 1.4 hours on the first day and increases to 2.1 hours on the twelfth day. It is mainly excreted via the kidneys.[4]
Tipiracil causes Cmax (highest blood plasma concentrations) of trifluridine to increase 22-fold, and its area under the curve 37-fold, by inhibiting thymidine phosphorylase.[4]
Chemistry
The substance is a white crystalline powder. It is freely soluble in methanol and acetone; soluble in water, ethanol, 0.01 M hydrochloric acid, and 0.01 M sodium hydroxide; sparingly soluble in isopropyl alcohol and acetonitrile; slightly soluble in diethyl ether; and very slightly soluble in isopropyl ether.[5]
References
- ↑ Long, Sarah S.; Pickering, Larry K.; Prober, Charles G. (2012). Principles and Practice of Pediatric Infectious Disease. Elsevier Health Sciences. p. 1502. ISBN 1437727026.
- 1 2 3 4 Drugs.com: Monograph for Trifluridine.
- ↑ Wilhelmus KR (2010). "Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis". Cochrane Database Syst Rev. 12: CD002898. doi:10.1002/14651858.CD002898.pub4. PMC 4739528
 . PMID 21154352.
. PMID 21154352. - 1 2 3 Haberfeld, H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
- ↑ FDA Professional Drug Information on Lonsurf.
External links
- Costin D, Dogaru M, Popa A, Cijevschi I (2004). "Trifluridine therapy in herpetic in keratitis". Rev Med Chir Soc Med Nat Iasi. 108 (2): 409–12. PMID 15688823.
- Kuster P, Taravella M, Gelinas M, Stepp P (1998). "Delivery of trifluridine to human cornea and aqueous using collagen shields.". CLAO J. 24 (2): 122–4. PMID 9571274.
- O'Brien W, Taylor J (1991). "Therapeutic response of herpes simplex virus-induced corneal edema to trifluridine in combination with immunosuppressive agents.". Invest Ophthalmol Vis Sci. 32 (9): 2455–61. PMID 1907950.
- "Trifluridine Ophthalmic Solution, 1%" (PDF). Retrieved 2007-03-24.