1,4-Dichlorobut-2-ene
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,4-Dichlorobut-2-ene | |
| Other names
trans-1,4-Dichlorobutene | |
| Identifiers | |
| 110-57-6 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChemSpider | 14394 |
| ECHA InfoCard | 100.003.437 |
| EC Number | 203-779-7 |
| |
| |
| Properties | |
| C4H6Cl2 | |
| Molar mass | 124.99 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.13 g/mL |
| Melting point | 1 °C (34 °F; 274 K) |
| Boiling point | 125.5 °C (257.9 °F; 398.6 K) |
| Hazards[1] | |
| GHS pictograms |  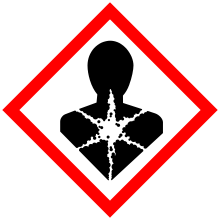 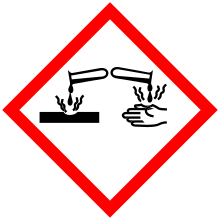 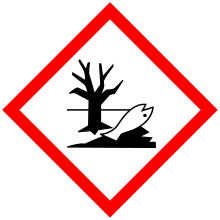 |
| GHS signal word | DANGER |
| H350, H330, H311, H301, H314, H410 | |
| EU classification (DSD) |
Carc. Cat. 2 Very toxic (T+) Corrosive (C) Dangerous for the environment (N) |
| R-phrases | R45-R24/25-R26-R34-R50/53 |
| S-phrases | S53-S45-S60-S61 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
1,4-Dichlorobut-2-ene is a chlorinated butene. It is an intermediate in the industrial production of chloroprene, and the main impurity in technical grade chloroprene.[2] The (E)-isomer is also one of the starting materials for Birman's total synthesis of the poriferic natural product sceptrin.[3]
Production of chloroprene
Chloroprene is a monomer for the production of synthetic rubbers such as Neoprene. It is produced from butadiene in a three-step process. The first step is the liquid- or vapour-phase chlorination of butadiene to a mixture of 3,4-dichlorobut-1-ene and 1,4-dichlorobut-2-ene (both isomers). In the second step, the mixture of 1,4-dichlorobut-2-ene and 3,4-dichlorobut-1-ene is isomerized to pure 3,4-dichlorobut-1-ene by heating to temperatures of 60–120 °C in the presence of a catalyst. Finally, dehydrochlorination (elimination of hydrogen chloride) of 3,4-dichlorobut-1-ene with dilute sodium hydroxide solution in the presence of polymerization inhibitors gives crude chloroprene.[4]
References
- ↑ Index no. 602-073-00-X of Annex VI, Part 3, to Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJEU L353, 31.12.2008, pp 1–1355 at p 473.
- ↑ Re-evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide (PDF), IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 71, Lyon, France: International Agency for Research on Cancer, 1999, pp. 227–50, ISBN 92-832-1271-1.
- ↑ Birman, Vladimir B.; Jiang, Xun-Tian (2004), "Synthesis of Sceptrin Alkaloids", Org. Lett., 6 (14): 2369–71, doi:10.1021/ol049283g, PMID 15228281. Baran, Phil S.; Zografos, Alexandros L.; O'Malley, Daniel P. (2004), "Short Total Synthesis of (±)-Sceptrin", J. Am. Chem. Soc., 126 (12): 3726–27, doi:10.1021/ja049648s, PMID 15038721.
- ↑ Kleinschmidt, P. (1986), "Chlorinated hydrocarbons. 6.4. 2-Chloro-1,3-butadiene", in Gerhartz, W.; Yamamoto, Y. S., Ullmann’s Encyclopedia of Industrial Chemistry, A6 (5th ed.), Weinheim: VCH, pp. 315–18. Stewart, C. A., Jr. (1993), "Chlorocarbons, -hydrocarbons (chloroprene)", in Kroschwitz, J. I.; Howe-Grant, M., Kirk-Othmer Encyclopedia of Chemical Technology, 6 (4th ed.), New York: John Wiley, pp. 70–78.