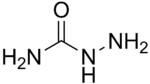
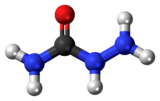
Semicarbazide
 | |
 | |
| Identifiers | |
|---|---|
| 57-56-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:28306 |
| ChEMBL | ChEMBL903 |
| ChemSpider | 5008 |
| ECHA InfoCard | 100.000.308 |
| KEGG | C02077 |
| PubChem | 5196 |
| |
| |
| Properties | |
| H2NNHC(=O)NH2 | |
| Molar mass | 75.08 g/mol |
| Melting point | 96 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Semicarbazide is the chemical compound with the formula OC(NH2)(N2H3). It is a water-soluble white solid. It is a derivative of urea.
Synthesis
The compound prepared by treating urea with hydrazine:[1]
- OC(NH2)2 + N2H4 → OC(NH2)(N2H3) + NH3
A further reaction can occur to give carbohydrazide:
- OC(NH2)(N2H3) + N2H4 → OC(N2H3)2 + NH3
Derivatives
Semicarbazide is frequently reacted with aldehydes and ketones to produce semicarbazones via a condensation reaction. This is an example of imine formation resulting from the reaction of a primary amine with a carbonyl group. The reaction is useful because semicarbazones, like oximes and 2,4-DNPs, typically have high melting points and crystallize, facilitating purification or identification of reaction products.[2]
Thiosemicarbazide is the analogous compoound with sulfur atom in place of oxygen atom,[3] with 4-Methyl-3-thiosemicarbazide being a simple example.
Properties
Semicarbazide products (semicarbazones and thiosemicarbazones) are known to have an activity of antiviral, antiinfective and antineoplastic through binding to copper or iron in cells.
Uses
Semicarbazide is used in preparing pharmaceuticals including: nitrofuran antibacterials (furazolidone, nitrofurazone, nitrofurantoin) and dizatrifone.
Semicarbazide is used as a detection reagent in thin layer chromatography (TLC). Semicarbazide stains α-keto acids on the TLC plate, which must then be viewed under ultraviolet light to see the results.
Occurrence
Semicarbazide has been shown to be formed in heat-treated flour containing azodicarbonamide as well as breads made from azodicarbonamide-treated flour.[4]
Structures

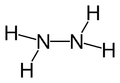
 Thiosemicarbazide
Thiosemicarbazide
See also
- Biurea - the conceptual dimer
- Carbazide - structurally related with the general formula (R2NNH)2C(O)
- Semicarbazide-cadmium therapy
References
- ↑ Jean-Pierre Schirmann, Paul Bourdauducq "Hydrazine" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a13_177.
- ↑ John McMurry (1984). Organic Chemistry. Brooks/Cole. p. 676.
- ↑ . PubChem https://pubchem.ncbi.nlm.nih.gov/compound/2723789. Missing or empty
|title=(help) - ↑ Becalski, Adam; Lau, Benjamin; Lewis, David; Seaman, Stephen (2004). "Semicarbazide Formation in Azodicarbonamide-Treated Flour: A Model Study". J. Agric. Food Chem. 52 (18): 5730. doi:10.1021/jf0495385.