Tetracycline-controlled transcriptional activation
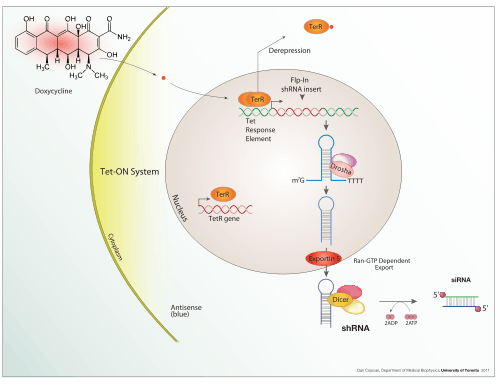
Tetracycline-Controlled Transcriptional Activation is a method of inducible gene expression where transcription is reversibly turned on or off in the presence of the antibiotic tetracycline or one of its derivatives (e.g. doxycycline).[1]
Tetracycline-controlled gene expression is based upon the mechanism of resistance to tetracycline antibiotic treatment found in gram-negative bacteria. In nature, the Ptet promoter expresses TetR, the repressor, and TetA, the protein that pumps tetracycline antibiotic out of the cell.[2]
The difference between Tet-On and Tet-Off is not whether the transactivator turns a gene on or off, as the name might suggest; rather, both proteins activate expression. The difference relates to their respective response to tetracycline or doxycycline (Dox, a more stable tetracycline analogue); Tet-Off activates expression in the absence of Dox, whereas Tet-On activates in the presence of Dox.
Tet-Off and Tet-On
The two most commonly used inducible expression systems for research of eukaryote cell biology are named Tet-Off and Tet-On.[3] The Tet-Off system for controlling expression of genes of interest in mammalian cells was developed by Professors Hermann Bujard and Manfred Gossen at the University of Heidelberg and first published in 1992.[4]
The Tet-Off system makes use of the tetracycline transactivator (tTA) protein, which is created by fusing one protein, TetR (tetracycline repressor), found in Escherichia coli bacteria, with the activation domain of another protein, VP16, found in the Herpes Simplex Virus.[5]
The resulting tTA protein is able to bind to DNA at specific TetO operator sequences. In most Tet-Off systems, several repeats of such TetO sequences are placed upstream of a minimal promoter such as the CMV promoter. The entirety of several TetO sequences with a minimal promoter is called a tetracycline response element (TRE), because it responds to binding of the tetracycline transactivator protein tTA by increased expression of the gene or genes downstream of its promoter.
In a Tet-Off system, expression of TRE-controlled genes can be repressed by tetracycline and its derivatives. They bind tTA and render it incapable of binding to TRE sequences, thereby preventing transactivation of TRE-controlled genes.
A Tet-On system works similarly, but in the opposite fashion. While in a Tet-Off system, tTA is capable of binding the operator only if not bound to tetracycline or one of its derivatives, such as doxycycline, in a Tet-On system, the rtTA protein is capable of binding the operator only if bound by a tetracycline. Thus the introduction of doxycycline to the system initiates the transcription of the genetic product. The Tet-On system is sometimes preferred over Tet-Off for its faster responsiveness.
Tet-Off expression systems are also used in generating transgenic mice, which conditionally express gene of interest.
Tet-On Advanced and Tet-On 3G
The Tet-On Advanced transactivator (also known as rtTA2S-M2) is an alternative version of Tet-On that shows reduced basal expression, and functions at a 10-fold lower Dox concentration than Tet-Off. In addition, its expression is considered to be more stable in eukaryotic cells due to being human codon optimized and utilizing 3 minimal transcriptional activation domains. It was discovered in 2000 as one of two improved mutants by H. Bujard and his colleagues after random mutagenesis of the Tet Repressor part of the transactivator gene.[6] Tet-On 3G (also known as rtTA-V16[citation needed]) is similar to Tet-On Advanced but was derived from rtTA2S-S2 rather than rtTA2S-M2. It is also human codon optimized and composed of 3 minimal VP16 activation domains. However, the Tet-On 3G protein has 5 amino acid differences compared to Tet-On Advanced which appear to increase its sensitivity to Dox even further. Tet-On 3G is sensitive to 100-fold less Dox and is 7-fold more active than the original Tet-On.[7]
Other systems
Other systems such as the T-REx system by Life Technologies work in a different fashion.[8] The gene of interest is flanked by an upstream CMV promoter and two TetO2 sites. Expression of the gene of interest is repressed by the high affinity binding of TetR homodimers to each TetO2 sequences in the absence of tetracycline. Introduction of tetracycline results in binding of one tetracycline on each TetR homodimer followed by release of TetO2 by the TetR homodimers. Unbinding of TetR homodimers and TetO2 result in derepression of the gene of interest.
Tetracycline Response Element (TRE)
In the most commonly used plasmids, the tetracycline response element consists of 7 repeats of the 19bp bacterial TetO sequence ( TCCCTATCAGTGATAGAGA ) separated by spacer sequences (for example: ACGATGTCGAGTTTAC ). It is the TetO that is recognized and bound by the TetR portion of Tet-On or Tet-Off. The TRE is usually placed upstream of a minimal promoter that has very low basal expression in the absence of bound Tet-Off (or Tet-On).
Advantages and Disadvantages
The Tet system has advantages over Cre, FRT, and ER (estrogen receptor) conditional gene expression systems. In the Cre and FRT systems, activation or knockout of the gene is irreversible once recombination is accomplished, whereas, in Tet and ER systems, it is reversible. The Tet system has very tight control on expression, whereas ER system is somewhat leaky.[9] However, the Tet system, which depends on transcription and subsequent translation of a target gene, is not as fast-acting as the ER system, which stabilizes the already-expressed target protein upon hormone administration. Also, since the 19bp tet-o sequence is naturally absent from mammalian cells, pleiotropy is thought to be minimized compared to hormonal methods of control. When using the Tet system in cell culture, it is important to confirm that each batch of fetal bovine serum is tested to confirm that contaminating tetracyclines are absent or are too low to interfere with inducibility.
The mechanism of action for the antibacterial effect of tetracyclines relies on disrupting protein translation in bacteria, thereby damaging the ability of microbes to grow and repair; however protein translation is also disrupted in eukaryotic mitochondria leading to effects that may confound experimental results.[10][11]
External links
- http://www.zmbh.uni-heidelberg.de/Bujard/default.shtml Hermann Bujard Lab, ZMBH, Heidelberg, Germany
- https://www.youtube.com/watch?v=a4WR6K6gPgw Tet-On Advanced Animation in YouTube
- http://jaxmice.jax.org/research/tet_intro.html (dead link) Jackson Laboratory
- http://www.la-press.com/tetracycline-regulated-systems-in-functional-oncogenomics-article-a200 A detailed overview of Tet-systems in functional cancer research and oncogenomics
See also
References
- ↑ Gossen M, Freundlieb S, Bender G, Müller G, Hillen W, Bujard H (1995). "Transcriptional activation by tetracyclines in mammalian cells". Science (journal). 268 (5218): 1766–1769. doi:10.1126/science.7792603. PMID 7792603.
- ↑ Orth P, Schnappinger D, Hillen W, Saenger W, Hinrichs W (2000). "Structural basis of gene regulation by the tetracycline inducible Tet repressor-operator system" (PDF). Nature Structural & Molecular Biology. 7 (3): 215–219. PMID 10700280.
- ↑ Tet-On and Tet-Off are registered trademarks of Clontech Laboratories, Inc. in the United States.
- ↑ Bujard, Hermann; M. Gossen (1992). "Tight Control of Gene Expression in Mammalian Cells by Tetracycline-Responsive Promoters.". Proc. Natl. Acad. Sci. U.S.A. 89 (12): 5547–51. doi:10.1073/pnas.89.12.5547. PMC 49329
 . PMID 1319065.
. PMID 1319065. - ↑ Allen, Nicholas D.; Antonius Plagge; Gavin Kelsey (2000). "Directed Mutagenesis in Embryonic Stem Cells". Mouse Genetics and Transgenics: 259–263.
- ↑ Urlinger, Stefanie; Baron, Udo; Thellmann, Marion; Hasan, Mazahir T.; Bujard, Herman; Hillen, Wolfgang (2000). "Exploring the sequence space for tetracycline-dependent transcriptional activators: Novel mutations yield expanded range and sensitivity.". Proc. Natl. Acad. Sci. U.S.A. 97 (14): 7963–8. doi:10.1073/pnas.130192197. PMC 16653
 . PMID 10859354.
. PMID 10859354. - ↑ Zhou, X.; Vink, M.; Klave, B.; Berkhout, B.; Das, A.T. (2006). "Optimization of the Tet-On system for regulated gene expression through viral evolution.". Gene Ther. 13 (19): 1382–1390. doi:10.1038/sj.gt.3302780. PMID 16724096.
- ↑ http://www.lifetechnologies.com/ca/en/home/references/protocols/proteins-expression-isolation-and-analysis/protein-expression-protocol/inducible-protein-expression-using-the-trex-system.reg.us.html/
- ↑ Sohal DS, Ngheim M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penniger JM, Molkentin JD (2001) Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using tamoxifen-inducible Cre protein. Circ Res 89:20-25
- ↑ Moullan N, Mouchiroud L, Wang X, Ryu D, Williams EG, Mottis A, Jovaisaite V, Frochaux MV, Quiros PM, Deplancke B, Houtkooper RH, Auwerx J (2015). "Tetracyclines Disturb Mitochondrial Function across Eukaryotic Models: A Call for Caution in Biomedical Research.". Celll Reports. 10 (10): 1681–91. doi:10.1016/j.celrep.2015.02.034. PMID 25772356.
- ↑ Chatzispyrou IA, Held NM, Mouchiroud L, Auwerx J, Houtkooper RH (2015). "Tetracycline antibiotics impair mitochondrial function and its experimental use confounds research.". Cancer Research. 72 (21): 4446–9. doi:10.1158/0008-5472.CAN-15-1626. PMID 26475870.