Tetraethyl pyrophosphate
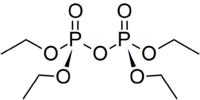 | |
 | |
| Names | |
|---|---|
| IUPAC name
tetraethyl diphosphate | |
| Identifiers | |
| 107-49-3 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL293787 |
| ChemSpider | 7585 |
| ECHA InfoCard | 100.003.179 |
| PubChem | 7873 |
| UNII | 28QKT80KX2 |
| |
| |
| Properties | |
| C8H20O7P2 | |
| Molar mass | 290.19 g·mol−1 |
| Appearance | colorless to amber liquid[1] |
| Odor | faint, fruity[1] |
| Density | 1.19 g/mL (20°C)[1] |
| Melting point | 0 °C; 32 °F; 273 K [1] |
| Boiling point | decomposes[1] |
| miscible[1] | |
| Vapor pressure | 0.0002 mmHg (20°C)[1] |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
| LDLo (lowest published) |
0.5 mg/kg (rat, oral) 2.3 mg/kg (guinea pig, oral) 3 mg/kg (mouse, oral)[2] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 0.05 mg/m3 [skin][1] |
| REL (Recommended) |
TWA 0.05 mg/m3 [skin][1] |
| IDLH (Immediate danger) |
5 mg/m3[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Tetraethyl pyrophosphate, abbreviated TEPP, is an organophosphate compound, which is used as an insecticide. This compound is a clear, colorless liquid, which is soluble in water, but hydrolyzes rapidly.[3] There are several mechanism by which TEPP can be synthesized and it was first synthesized by Philippe de Clermont and Muscovite Wladimir Moschnin in 1854. It is a toxic compound, due to its anticholinesterase activity, which not only applies to insects, but also to other animals. TEPP is metabolized with the assistance of many different enzymes en the metabolistes are excreted via the urine.
History
The first reported synthesis of tetraethyl pyrophosphate was in the year 1854.[4] Muscovite Wladimir Moschnin and Philippe de Clermont were the first chemists who synthesized and reported the TEPP synthesis. At that time, both were working at Adolphe Wurtz' laboratory in Paris. The fact that nobody knew anything about the toxicity of TEPP at this time was evidenced by De Clermont himself, who reported that he had tasted the compound out of curiosity. He described the taste as being a viscous liquid with a burning taste and a peculiar odor ("un liquide visqueux d’une saveur brulante(et) d’une odeur particuliere").[5] Even though TEPP has repeatedly been synthesized by other chemists during the years that followed, not until the 1930s had any adverse effects been observed. Furthermore, Philippe de Clermont has never been reported ill by his family up to his passing at the age of 90. In the meantime, organophosphorus chemistry has really started developing with the help of A. W. von Hofmann, Carl Arnold August Michaelis and Alecsandr Erminingel'dovich Arbusow.[6]
It was not until 1932 before the first adverse effects of compounds similar to TEPP had been recognized. Willy Lange and Gerda von Krueger were the first to report such effects, about which the following statement was published in their article (in German):[7]
"Interestingly, we report the strong effect of monofluorophosphate phosphoric acid alkyl esters on the human organism. The vapor of these compounds have a pleasant odor and sharply aromatic. After only a few minutes of inhaling the vapor, there is a strong pressure on the larynx, associated with shortness of breath. Then comes decreased awareness, opacities, and dazzling phenomena causing painful sensitivity of the eye to light. Only after several hours is there relief from these phenomena. They are apparently not caused by acidic decomposition products of the ester, but they are probably due to the Dialkyl monofluorophosphates themselves. The effects are exerted by very small amounts. "
Starting in 1935 the German government started gathering information about new toxic substances, of which some were to be classified as secret by the German Ministry of Defence.[6] Gerhard Schrader, who has become famous for his studies into organophosphorus insecticides and nerve gasses, was one of the chemists who was also studying TEPP. In his studies, in particular his studies into the biological aspects, he noticed that this reagent could possibly be used as an insecticide. This would make the classification of the compound as secret disadvantageous for commercial firms .[6]
Around the beginning of the Second World War, several independent studies were conducted by the military and pharmaceutical companies. It was discovered that TEPP is an inhibitor of cholinesterases.[5] Schrader referred to the studies by Eberhard Gross, who was the first to recognize the mechanism of action for TEPP in 1939. More experiments were conducted by other pharmacologists such as Hans Gremels, who was able to confirm Gross’s work a year later.[6] Gremels was also involved in the development of nerve gasses at that time. The results followed from a study in which different species of animals and several human volunteers were involved. Around that same time, atropine was discovered as a possible antidote for the anticholinesterase activity of TEPP.
After the Second World War, Schrader was among many German scientists who were interrogated by English scientists, among others. During the war, the English had been developing chemical weapons of their own to surprise their enemies. In these interrogations the existence of TEPP and other insecticides were disclosed. The existence of nerve gasses, however also being disclosed by Schrader, was kept secret by the military.[8]
Structure and reactivity
Tetraethyl pyrophosphate is a very toxic organophosphorus ester. In its pure form it is a colourless, hygroscopic liquid. It is fully miscible with water and various types of organic solvents, except for petroleum based solvents. The liquid evaporates very slowly and hydrolyses very rapidly in water. Because the mechanism for the hydrolysis is first order, the half-lifes were determined to be 7.5 hours at 25 °C and 3.1 hours at 40 °C when dissolved in distilled water. If one heats TEPP it will start to decompose at around 150-200 °C, which produces ethylene gas. Furthermore, acidic fumes of phosphoric acid and carbon monoxide could be formed.[9] At lower temperatures TEPP could form triethylphosphate and ethyl metaphosphates, which are acid catalysed reactions.[10]
As an insecticide TEPP is degraded to water soluble, often non-toxic compounds. It takes one to three days for TEPP to decompose, which is usually the case for organophosphor-based insecticides. Furthermore, the concentration in soil decreases exponentially by both erosion as chemical or biological degradation.[11]
Synthesis
The synthesis by De Clermont and Moschnin was based on the earlier work by Alexander Williamson (who is well known for the Williamson-synthesis of ethers).[5] In their synthesis, they made use of ethyl iodide and silver salts to form esters in combination with pyrophosphate. The reaction mechanism shows the silver of the pyrophosphoric acid being alkylated with ethyl iodide.[12] Silver iodide was used as a catalyst for this reaction.
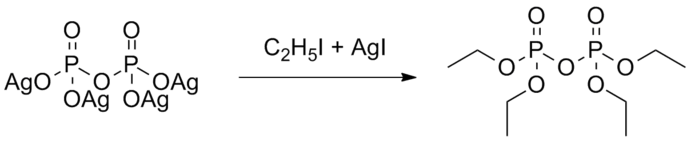
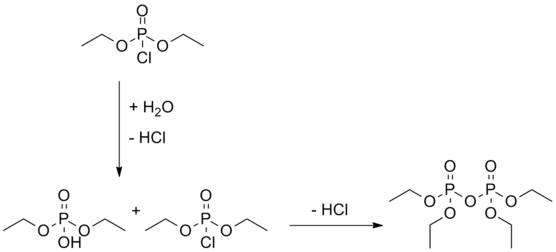
In the commercial preparation of tetraethyl pyrophosphate, it is common to use the methods developed by Schrader, Woodstock or Toy. One can carry out the synthesis by reacting triethyl phosphate with phosphorus oxychloride (Schrader-method) or with phosphorus pentoxide (Woodstock-method).[10] One could also synthesize the pure substance by controlled hydrolysis of diethyl phosphorochloridate in a mixture of water and pyridine.[8]
Mechanism of action
Organophosphates like TEPP inhibit the function of cholinesterases, which are important enzymes for the degradation of the neurotransmitter acetylcholine.

In the active centre of cholinesterases, there are two important sites, namely the anionic site and the esteratic site. The anionic site has a negative charge and can bind the quaternary nitrogen of acetylcholine, which has a positive charge.
After the binding of acetylcholine to the anionic site of the cholinesterase, the acetyl group of acetylcholine can bind to the esteratic site. The most important amino acid residues in the esteratic site are a glutamate, a histidine and a serine. These residues mediate the hydrolysis of the acetylcholine molecule.
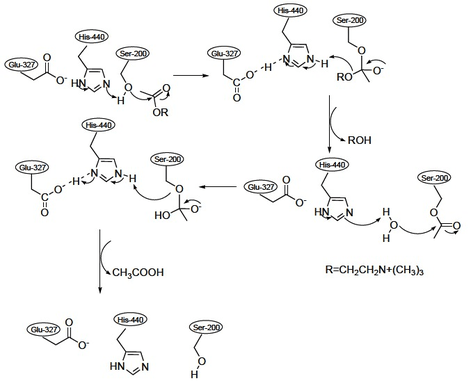
At the esteratic site the acetylcholine is cleaved, which results in a free choline moiety and an acetylated cholinesterase. In this state the cholinesterase isn’t capable of degrading acetylcholine molecules. Therefore, the acetyl group is hydrolysed to acetate and it will dissociate from the cholinesterase. Now the cholinesterase is functional again and it can degrade other acetylcholine molecules. This results in a decrease in response of the acetylcholine receptor.[13][14]
However, when TEPP is present it will bind the serine residue in the esteratic site of the cholinesterase. Later on the TEPP molecule will be transformed into a single phosphate molecule.

In this way the cholinesterase is being phosphorylated. This phosphorylation inhibits the binding of the acetyl group of the acetylcholine to the esteratic site of the cholinesterase. Because the acetyl group can’t bind the cholinesterase, the acetylcholine can’t be cleaved. Therefore the acetylcholine will remain intact and will accumulate in the synapses. This results in continuous activation of acetylcholine receptors, which leads to the acute symptoms of TEPP poisoning.[15] The phosphorylation of cholinesterase by TEPP (or any other organophosphate) is irreversible. This makes the inhibition of the cholinesterase permanent.[13][14]
The cholinesterase gets irreversible phosphorylated according to the following reaction scheme
In this reaction scheme the E indicates the cholinesterase, PX the TEPP molecule, E*PX the reversible phosphorylated cholinesterase, k3 the reaction rate of the second step, EP the phosphorylated cholinesterase and X the leaving group of the TEPP.
The irreversible phosphorylation of the cholinesterase occurs in two steps. In the first step the cholinesterase gets reversibly phosphorylated. This reaction is very fast. Then the second step takes place. The cholinesterase forms a very stable complex with TEPP, in which TEPP is covalently bound to the cholinesterase. This is a slow reaction. But after this step the cholinesterase is irreversibly inhibited.[13]
The time dependent irreversible inhibition of the cholinesterase can be described by the following equation.[13]

In this formula, E is the remaining enzyme activity, E0 is the initial enzyme activity, t is the time interval after mixing of the cholinesterase and the TEPP, KI is the dissociation constant for cholinesterase-TEPP complex (E*PX) and I is the TEPP concentration.
The reaction mechanism and the formula above are both also compatible for other organophosphates. The process occurs in the same way.
Furthermore, certain organophosphates can cause OPIDN, organophosphate-induced delayed polyneuropathy. This is a disease, which is characterized by degeneration of axons in the peripheral and central nervous system. This disease will show a few weeks after contamination with the organophosphate. It is believed that the neuropathy target esterase (NTE) is affected by the organophosphate which induces the disease. However, there are no references found, which indicate that TEPP is one of the organophosphates that can cause OPIDN.[16]
Metabolism
The metabolism of organophosphates can result in either the production of more toxic compounds (toxification) or less toxic compounds (detoxification). TEPP will most likely be detoxificated into less toxic metabolites.[17]
TEPP and most of the other organophosphates are detoxified by oxidation and hydrolysis.[18] Due to this oxidation and hydrolysis the compound gets more polar, which makes it much easier to excrete via the urine. Note that TEPP is a lipophilic compound, so it can diffuse through tissues easily. This makes it hard to excrete TEPP.
Many enzymes are important for the degradation of TEPP. One of these are the phosphotriesterases (PTEs). In the serum and the liver, there is a significant higher PTE activity found than in other tissues of mammals.[18]
PTEs are responsible for the cleavage of the bond between the phosphorus atom and the leaving group. This reaction takes place in combination with water. When TEPP is broken down, the product will be diethyl phosphate, which is more polar than the original TEPP molecule. Due to the more polar character of the diethyl phosphate, it will diffuse and accumulate less into fatty tissues and it can be eliminated more easily via the urine. Furthermore, the diethyl phosphate is less able to phosphorylate other molecules. This is the reason why it is less toxic.[17][18] This reaction is an example of a phase I reaction.[19] After this phase I reaction it is most likely that other phase I reactions will take place to make the metabolites more soluble. The OH-group will probably be transformed into a COOH-group to make the molecule even more polar.
After the phase I reactions, a phase II reaction will take place. An endogenous compound, like glucuronides, sulfates, acetates and amino acids, will be conjugated to the metabolites to make it even more water-soluble. Then the complex can be excreted via the urine.[19]
Toxicity
Lethality
Acute toxicity, deadly doses:
- DERMAL: LD50 = 2.4 mg/kg (male rat)
- ORAL: LD50 = 1.12 mg/kg (rat)[9]
So the toxicity of TEPP falls in category 1, which means that it requires the least amount of exposure to be lethal.
It has been reported that the lethal oral dose is 1.429 mg/kg.[20] An oral dose of 1.429 mg/kg is equivalent to a 70-kg worker being exposed to 67 mg/m3 for 30 minutes assuming a 50 liter per minute breathing rate and 100% absorption.
TEPP can cause death within hours or even minutes, but also can be delayed for several days. When it is given in small doses, humans could survive a total dose of 40 mg/kg.[21]
An injection with TEPP (~0.01 mg/kg, or more) can result in a fast depression of acetylcholinesterase in the plasma and the blood. When TEPP is taken orally, a dose of approximately four times larger is needed to induce the same effect.
Considerations for environment
TEPP is very quickly metabolised by animals, and therefore dangerous. It is moderately dangerous to fish, birds, insects and wildlife.[9] It is unlikely that there is a biological magnification.
Emergency and first aid procedures[9]
When someone is intoxicated by TEPP, only when it is only suspected, one should handle very quickly. Help must be sought in the form of a doctor, hospital or poison control centre.
Frequent early symptoms of tetraethyl pyrophosphate poisoning are dizziness, headache, weakness, muscle twitching, tremor, abdominal cramps, nausea, diarrhea and sweating can develop within 4–12 hours of the contact.
Blurred or dark vision, tightness in the chest, confusion, wheezing and productive coughing may occur.
Slowing of the heartbeat rarely progresses to complete sinus arrest, but the respiratory depression can be fatal.
Severe poisoning could be indicated by incontinence, unconsciousness and convulsions. If there is excess to atropine antidote for the anticholinesterase activity of TEPP.
First aid procedures
SKIN CONTACT: If there is any chance that skin and/or hair are contaminated, you should bathe and shampoo the victim with soap and water.
INGESTION: If the victim is alert and the respiration is not depressed, Syrup of Ipecac should be given, followed by 1-2 glasses of water to induce vomiting. Adults: 30mL; children: 15mL.
INHALATION: If one breathes in large amounts of TEPP, bring the exposed person immediately to fresh air. If the breathing has stopped, perform artificial respiration. Keep the affected person warm and at rest. Get medical attention as soon as possible.
EYE CONTACT: Wash eyes immediately with large amounts of water, lifting the lower and upper lids occasionally. Get medical attention as soon as possible. Contact lenses should not be worn when working with this chemical.
Efficacy and effects
Efficacy
TEPP has shown to be a very good insecticide to pests like aphids, mites, spiders, mealybugs, leafhoppers, lygus bugs, thrips, leafminers, and many others.[9] TEPP and other organophosphates are the most widely used pesticides in the U.S. due to their effectiveness and relative small impact on the environment.[22] Organophosphates are hydrolyzed and broken down so easily, that the residual period is approximately 48 hours for TEPP.[9] That is enough time for TEPP to do its work, since TEPP is mostly acute toxic with its nerve gas-like properties.[22]
Effects on laboratory animals
Not only is TEPP very toxic for humans, it is also highly toxic for warm-blooded animals. This includes direct contact and inhalation of the vapors.[23] There are three types of effects on these animals that have come forward during laboratory studies.
There are the muscarinic signs, which are effects that are similar to stimulation on postganglionic parasympathic nerves. These signs are usually the first to manifest. They consist of hypersalivation, the secretion of tears, sweating, and nasal discharge. Dilation of the pupil (miosis), impaired breathing (dyspnea), vomiting, diarrhea, and frequency of urination are also signs in that may occur in this category.
The nicotinic effects are those that are related to the acetylcholine receptors in the nervous system. These effects contain fasciulation (uncontrollable twitches) of the muscles, weakness and paralysis.
The last category is the central nervous system. The effects consist of nervousness, apprehension, ataxia, convulsions and ending up into a coma.
Finally death is mostly due to either respiratory failure and in some cases cardiac arrest. The route of absorption might be responsible for the range of effect on certain systems.[24]
For cold-blooded animals the effects are slightly different. In a study with frogs, acute exposure caused a depression in the amount of erythocytes in the blood. There was also a reduction of white bloodcells, especially the neutrophil granulocytes and lymphocytes. There was no visible damage to the bloodvessels to explain the loss of blood cells. Further there were no signs like hypersalivation or tears like in warm-blooded animals. Though there was hypotonia leading to paralysis.[25]
Therapeutic usage
TEPP has not only been used as a pesticide, but also as a treatment for myasthenia gravis. This autoimmune disease is known for its grave muscle weakness. The treatment would deliver an increase in strength. The amount of TEPP had to be very exact. The range for effectiveness of the dose is between 2–4 mg above or under the ideal dose, with maximum response and without any side effects. Further ranges the differences between a dose with the maximum response and toxicity between 0.5–3 mg.[26]
References
- 1 2 3 4 5 6 7 8 9 10 "NIOSH Pocket Guide to Chemical Hazards #0590". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "TEPP". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ Robert L. Metcalf (2005), "Insect Control", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a14_263
- ↑ Fest, Christa; Schmidt, Karl-Julius. The Chemistry of Organophosphorus Pesticides - Springer. doi:10.1007/978-3-642-68441-8.
- 1 2 3 Petroianu, G. A. (2009-04-01). "The synthesis of phosphor ethers: who was Franz Anton Voegeli?". Die Pharmazie. 64 (4): 269–271. doi:10.1691/ph.2009.8244. ISSN 0031-7144. PMID 19435147.
- 1 2 3 4 "9: "Structure-Activity Relationships of the Organophosphorus Anticholinesterase Agents, Historical development of organophosphorus cholinesterase inhibitors." Handbook of Experimental Pharmacology.". Cholinesterases and Anticholinesterase Agents. 15. Springer Science & Business Media. 1963. pp. 434–437. ISBN 978-3-642-99875-1.
- ↑ Petroianu, G. A. (2010-10-01). "Toxicity of phosphor esters: Willy Lange (1900-1976) and Gerda von Krueger (1907-after 1970)". Die Pharmazie. 65 (10): 776–780. ISSN 0031-7144. PMID 21105582.
- 1 2 Toy, A. D. F. (1948). "The Preparation of Tetraethyl Pyrophosphate and Other Tetraalkyl Pyrophosphates". J. Am. Chem. Soc. 70 (11): 3882–3886. doi:10.1021/ja01191a104.
- 1 2 3 4 5 6 "TEPP (Kilmite 40) - Chemical Profile 3/85". pmep.cce.cornell.edu. Retrieved 2016-03-08.
- 1 2 Sherma, Joseph; Zweig, Gunter (1973). Thin-Layer and Liquid Chromatography and Pesticides of International Importance: Analytical Methods for Pesticides and Plant Growth Regulators. 7. Academic Press. pp. 471–477. ISBN 978-1-4832-2084-0.
- ↑ National Research Council (U S. ) Pesticide Residues Committee (1965). Report on "no Residue" and "zero Tolerance". National Academies. pp. 3–4.
- ↑ "History of organophosphorus cholinesterase inhibitors & reactivators". Military Medical Science Letters. 84 (4): 182–185. 2015.
- 1 2 3 4 5 6 Čolović, Mirjana B.; Krstić, Danijela Z.; Lazarević-Pašti, Tamara D.; Bondžić, Aleksandra M.; Vasić, Vesna M. (2013-05-01). "Acetylcholinesterase Inhibitors: Pharmacology and Toxicology". Current Neuropharmacology. 11 (3): 315–335. doi:10.2174/1570159X11311030006. ISSN 1570-159X. PMC 3648782
 . PMID 24179466.
. PMID 24179466. - 1 2 O'Brien, Richard D. (2013-10-22). Toxic Phosphorus Esters: Chemistry, Metabolism, and Biological Effects. Elsevier. ISBN 978-1-4832-7093-7.
- ↑ Roberts, Stephen M.; James, Robert C.; Williams, Phillip L. (2014-12-08). Principles of Toxicology: Environmental and Industrial Applications. John Wiley & Sons. ISBN 978-1-118-98248-8.
- ↑ Lotti, Marcello; Moretto, Angelo (2005-01-01). "Organophosphate-induced delayed polyneuropathy". Toxicological Reviews. 24 (1): 37–49. doi:10.2165/00139709-200524010-00003. ISSN 1176-2551. PMID 16042503.
- 1 2 O'Brien, R. D. (2014-06-28). Insecticides: Action and Metabolism. Academic Press. ISBN 9781483270685.
- 1 2 3 Sogorb, Miguel A; Vilanova, Eugenio (2002-03-10). "Enzymes involved in the detoxification of organophosphorus, carbamate and pyrethroid insecticides through hydrolysis". Toxicology Letters. 128 (1–3): 215–228. doi:10.1016/S0378-4274(01)00543-4.
- 1 2 Jokanović, Milan (2001-09-25). "Biotransformation of organophosphorus compounds". Toxicology. 166 (3): 139–160. doi:10.1016/S0300-483X(01)00463-2.
- ↑ "CLINICAL MEMORANDA ON ECONOMIC POISONS". California Medicine. 86 (6): 428. 1957-06-01. ISSN 0008-1264. PMC 1511953
 .
. - ↑ Roach, S. A.; Rappaport, S. M. (1990-01-01). "But they are not thresholds: A critical analysis of the documentation of threshold limit values". American Journal of Industrial Medicine. 17 (6): 727–753. doi:10.1002/ajim.4700170607. ISSN 1097-0274.
- 1 2 "Organophosphates". Toxipedia. Gilbert, S. Retrieved 2016-02-28.
- ↑ "BENFLURALIN - National Library of Medicine HSDB Database". toxnet.nlm.nih.gov. Retrieved 2016-03-08.
- ↑ Clarke, Myra L.; Harvey, Douglas Graham; Humphreys, David John (1988). Veterinary Toxicology (2 ed.). London, England: Bailliere Tindal. p. 157.
- ↑ Kaplan, Harold M.; Glaczenski, Sheila S. (1965-06-01). "Hematological effects of organophosphate insecticides in the frog (Rana pipiens)". Life Sciences. 4 (12): 1213–1219. doi:10.1016/0024-3205(65)90335-8.
- ↑ "TEPP | C8H20O7P2 - PubChem". pubchem.ncbi.nlm.nih.gov. Retrieved 2016-03-08.