Strecker amino acid synthesis
| Strecker synthesis | |
|---|---|
| Named after | Adolph Strecker |
| Reaction type | Substitution reaction |
| Identifiers | |
| Organic Chemistry Portal | strecker-synthesis |
| RSC ontology ID | RXNO:0000207 |
The Strecker amino acid synthesis, also known simply as the Strecker synthesis, was devised by German chemist Adolph Strecker, and is a term used for a series of chemical reactions that synthesize an amino acid from an aldehyde or ketone.[1][2][3] The aldehyde is condensed with ammonium chloride in the presence of potassium cyanide to form an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino acid.[4][5] In the original Strecker reaction acetaldehyde, ammonia, and hydrogen cyanide combined to form after hydrolysis alanine.
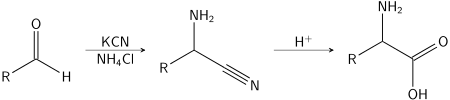
While usage of ammonium salts gives unsubstituted amino acids, primary and secondary amines also successfully give substituted amino acids. Likewise, the usage of ketones, instead of aldehydes, gives α,α-disubstituted amino acids.[6]
The traditional synthesis of Adolph Strecker from 1850 gives racemic α-amino nitriles, but several procedures utilizing asymmetric auxiliaries[7] or asymmetric catalysts[8][9] have been developed.[10]
Reaction mechanism
In the first part of the reaction, the carbonyl oxygen of an aldehyde is protonated, followed by a nucleophilic attack of ammonia to the carbonyl carbon. After subsequent proton exchange, water is cleaved from the iminium ion intermediate. A cyanide ion then attacks the iminium carbon yielding an aminonitrile.

In the second part of the Strecker Synthesis the nitrile nitrogen of the aminonitrile is protonated, and the nitrile carbon is attacked by a water molecule. A 1,2-diamino-diol is then formed after proton exchange and a nucleophilic attack of water to the former nitrile carbon. Ammonia is subsequently eliminated after the protonation of the amino group, and finally the deprotonation of a hydroxyl group produces an amino acid.

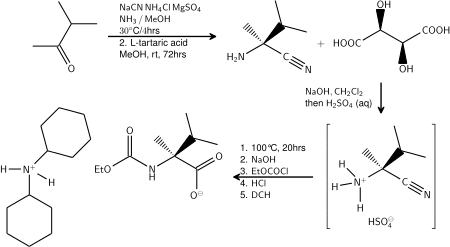
One example of the Strecker synthesis is a multikilogram scale synthesis of an L-valine derivative starting from 3-methyl-2-butanone:[11][12]
Asymmetric Strecker reactions
The asymmetric Strecker reaction was pioneered by Kaoru Harada in 1963.[13][14] By replacing ammonia with (S)-alpha-phenylethylamine as chiral auxiliary the ultimate reaction product was chiral alanine. The first asymmetric synthesis via a chiral catalyst was reported in 1996.[15]
Catalytic Asymmetric Strecker reactions
Catalytic asymmetric Strecker reaction can be effected using thiourea-derived catalyst.[16] In 2012, a BINOL-derived catalyst was employed to generate chiral cyanide anion.[17]

References
- ↑ Strecker, A. (1850). "Ueber die künstliche Bildung der Milchsäure und einen neuen, dem Glycocoll homologen Körper". Annalen der Chemie und Pharmacie. 75 (1): 27–45. doi:10.1002/jlac.18500750103.
- ↑ Strecker, A. (1854). "Ueber einen neuen aus Aldehyd – Ammoniak und Blausäure entstehenden Körper (p )". Annalen der Chemie und Pharmacie. 91 (3): 349–351. doi:10.1002/jlac.18540910309.
- ↑ Shibasaki, M.; Kanai, M.; Mita, K. Org. React. 2008, 70, 1. doi:10.1002/0471264180.or070.01
- ↑ Kendall, E. C.; McKenzie, B. F. Organic Syntheses, Coll. Vol. 1, p.21 (1941); Vol. 9, p.4 (1929). (Article)
- ↑ Clarke, H. T.; Bean, H. J. Organic Syntheses, Coll. Vol. 2, p.29 (1943); Vol. 11, p.4 (1931). (Article)
- ↑ Masumoto, S.; Usuda, H.; Suzuki, M.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2003, 125(19), 5634–5635. (doi:10.1021/ja034980+)
- ↑ Davis, F. A. et al. Tetrahedron Lett. 1994, 35, 9351.
- ↑ Ishitani, H.; Komiyama, S.; Hasegawa, Y.; Kobayashi, S. J. Am. Chem. Soc. 2000, 122(5), 762–766. (doi:10.1021/ja9935207)
- ↑ Huang, J.; Corey, E. J. Org. Lett. 2004, 6(26), 5027–5029. (doi:10.1021/ol047698w)
- ↑ Duthaler, R. O. Tetrahedron 1994, 50, 1539–1650. (Review, doi:10.1016/S0040-4020(01)80840-1)
- ↑ A Concise Synthesis of (S)-N-Ethoxycarbonyl—methylvaline Jeffrey T. Kuethe, Donald R. Gauthier, Jr., Gregory L. Beutner, and Nobuyoshi Yasuda J. Org. Chem., 72 (19), 7469 -7472, 2007. doi:10.1021/jo7012862
- ↑ The initial reaction product of 3-methyl-2butanone with sodium cyanide and ammonia is resolved by application of L-tartaric acid. The amino acid is isolated as its salt with dicyclohexylamine.
- ↑ Asymmetric Synthesis of α-Amino-acids by the Strecker Synthesis Kaoru Harada Nature 200, 1201 (21 December 1963); doi:10.1038/2001201a0
- ↑ Asymmetric Strecker Reactions Jun Wang, Xiaohua Liu, Xiaoming Feng 2011 Chemical Reviews Article ASAP doi:10.1021/cr200057t
- ↑ Asymmetric Catalysis of the Strecker Amino Acid Synthesis by a Cyclic Dipeptide Mani S. Iyer,, Kenneth M. Gigstad,, Nivedita D. Namdev, and, Mark Lipton Journal of the American Chemical Society 1996 118 (20), 4910–4911 doi:10.1021/ja952686e
- ↑ "Scaleable catalytic asymmetric Strecker syntheses of unnatural alpha-amino acids" doi:10.1038/nature08484 http://www.nature.com/nature/journal/v461/n7266/full/nature08484.html
- ↑ "Scalable organocatalytic asymmetric Strecker reactions catalysed by a chiral cyanide generator" doi:10.1038/ncomms2216 http://www.nature.com/ncomms/journal/v3/n11/full/ncomms2216.html