Silylene
 | |
| Names | |
|---|---|
| IUPAC name
Silylene | |
| Systematic IUPAC name
Silylidene | |
| Other names
Hydrogen silicide(-II) Silicene | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 4885758 |
| PubChem | 6327230 |
| |
| |
| Properties | |
| H2Si | |
| Molar mass | 30.10 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Silylenes are chemical compounds containing a divalent silicon atom without any electrical charge. Both dicoordinate and tricoordinate silylenes are reported in the literature. They are considered to be heavier analogues of carbene. In earlier times, they were called silene, but this is a mistake, as that term means compounds of π-bonded silicon: Si=C. The generic term analogous to carbene is "silicene."
Silylenes have been proposed as reactive intermediates and are so unstable that they usually cannot be isolated. While carbenes are observed in either the triplet or singlet state depending on the nature of the substituents, silylenes typically have a singlet ground state because the energy gap between the 3s and 3p orbitals of the silicon atom is very large and so the singlet-triplet gaps are enormous.
History
The first experimental chemical observation of silylene as an intermediate was demonstrated by P.S. Skell and E.J. Goldstein in 1964.
The first stable silylene was synthesized and isolated as a diamino silylene, N,N’-di-tert-butyl-1,3-diaza-2-silacyclopent-4-en-2-ylidene, in 1994 by M. Denk et al.[1]
Synthesis and properties
Silylenes are generally synthesized by thermolysis or photolysis of polysilanes, by silicon atom reactions (insertion, addition or abstraction), by pyrolysis of silanes, or by reduction of 1,1-dihalosilane.
Simple silylenes are very reactive species and easily dimerize to give disilenes, so their lifetime is very short. Bulky substituents are used to prevent this dimerization and stabilize silylenes effectively for long-term survival in dilute solution and even in crystals.
The reactivities of silylenes are similar to those of carbenes, and insertion to polar bonds, addition to multiple bonds, and dimerization occur. But, in contrast to carbenes, insertion to C-H bonds and C-C bonds generally does not happen and complexation to Lewis base takes place.
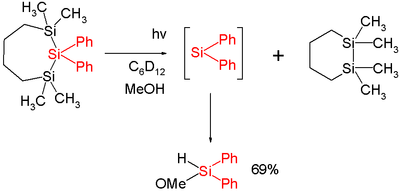
In one study diphenylsilylene is generated by flash photolysis of a trisilane:[2]
In this reaction diphenylsilylene is simply extruded with formation of the disilane. The silylene can be observed with UV spectroscopy at 520 nm and is short-lived with a chemical half-life of 2microseconds. Added methanol acts as a chemical trap with a second order rate constant of 13x109 mol−1s−1 which is close to diffusion control.
Persistent silylenes
Just as with persistent carbenes, silylenes can be stabilized. The first stable silylenes were discovered by Robert West at the University of Wisconsin–Madison. In one study [3] a stable silylene is synthesized by debromination the dibromosilyl compound 1 by potassium graphite:
The α-nitrogen atoms in 2 stabilize the electrophilic silicon atom by п-donation. In one contributing resonance structure 2b a negative charge is centered in an exocyclic methylene group and a positive charge delocalized over the silicon ring system. X-ray diffraction of the compound shows equal N-Si bonds (173 pm) and an unusually short exocylic bond with bond length of 143.6 pm. The compound reacts with trimethylsilyltriflate to the 1,4-adduct 3 (kinetic product) which slowly isomerizes to the 1,1-adduct 4 as the thermodynamically controlled product.
See also
- Carbene
- Silylium ions, protonated silylenes
References
- ↑ Denk, M., Green, J.C., Metzler, N., Wagner, M. (1994), Electronic structure of a stable silylene: photoelectron spectra and calculations. Journal of the Chemical Society, Dalton Transactions, 2405–2410.
- ↑ Diphenylsilylene Andrey G. Moiseev and William J. Leigh J. Am. Chem. Soc.; 2006; 128(45) pp 14442 - 14443; (Communication) doi:10.1021/ja0653223
- ↑ A New Type of N-Heterocyclic Silylene with Ambivalent Reactivity Matthias Driess, Shenglai Yao, Markus Brym, Christoph van Wüllen, and Dieter Lentz J. Am. Chem. Soc.; 2006; 128(30) pp 9628 - 9629; (Communication) doi:10.1021/ja062928i