Seyferth–Gilbert homologation
| Seyferth–Gilbert homologation | |
|---|---|
| Named after | Dietmar Seyferth John C. Gilbert |
| Reaction type | Homologation reaction |
| Identifiers | |
| Organic Chemistry Portal | seyferth-gilbert-homologation |
| RSC ontology ID | RXNO:0000387 |
The Seyferth–Gilbert homologation is a chemical reaction of an aryl ketone 1 (or aldehyde) with dimethyl (diazomethyl)phosphonate 2 and potassium tert-butoxide to give substituted alkynes 3.[1][2] Dimethyl (diazomethyl)phosphonate 2 is often called the Seyferth–Gilbert reagent.[3]

This reaction is called a homologation because the product has exactly one additional carbon more than the starting material.
Reaction mechanism
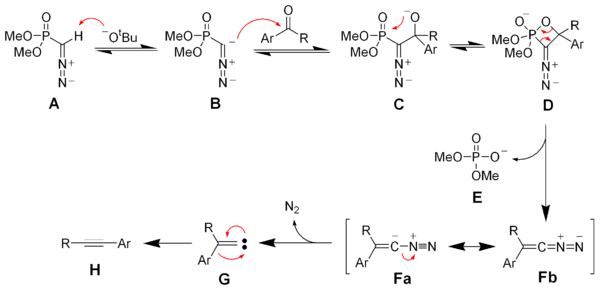
Deprotonation of the Seyferth–Gilbert reagent A gives an anion B, which reacts with the ketone to form the oxaphosphetane D. Elimination of dimethylphosphate E gives the vinyl diazo-intermediate Fa and Fb. The generation of nitrogen gas gives a vinyl carbene G, which via a 1,2-migration forms the desired alkyne H.
Bestmann modification
 | |
| Names | |
|---|---|
| IUPAC name
dimethyl (1-diazo-2-oxopropyl)phosphonate | |
| Identifiers | |
| 90965-06-3 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 9281325 |
| PubChem | 11106189 |
| |
| |
| Properties | |
| C5H9N2O4P | |
| Molar mass | 192.11 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dimethyl (diazomethyl)phosphonate can be generated in situ from dimethyl-1-diazo-2-oxopropylphosphonate (also called the Ohira-Bestmann reagent) by reaction with methanol and potassium carbonate. Reaction of Bestmann's reagent with aldehydes gives terminal alkynes often in very high yield.[4][5]
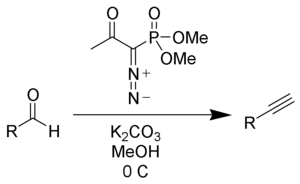
The use of the milder potassium carbonate makes this procedure much more compatible with a wide variety of functional groups.
Improved in situ generation of the Ohira-Bestmann reagent
Recently a safer and more scalable approach has been developed for the synthesis of alkynes from aldehydes. This protocol takes advantage of a stable sulfonyl azide, rather than tosyl azide, for the in situ generation of the Ohira−Bestmann reagent.[6]
Another modification for less reactive aldehydes is made by replacement of potassium carbonate with caesium carbonate in MeOH and results in a drastic yield increase.[7]
See also
References
- ↑ D. Seyferth, R. S. Marmor and P. Hilbert (1971). "Reactions of dimethylphosphono-substituted diazoalkanes. (MeO)2P(O)CR transfer to olefins and 1,3-dipolar additions of (MeO)2P(O)C(N2)R". J. Org. Chem. 36 (10): 1379–1386. doi:10.1021/jo00809a014.
- ↑ J. C. Gilbert & U. Weerasooriya (1982). "Diazoethenes: their attempted synthesis from aldehydes and aromatic ketones by way of the Horner-Emmons modification of the Wittig reaction. A facile synthesis of alkynes". J. Org. Chem. 47 (10): 1837–1845. doi:10.1021/jo00349a007.
- ↑ D. G. Brown, E. J. Velthuisen, J. R. Commerford, R. G. Brisbois and T. H. Hoye (1996). "A Convenient Synthesis of Dimethyl (Diazomethyl)phosphonate (Seyferth/Gilbert Reagent)". J. Org. Chem. 61 (7): 2540–2541. doi:10.1021/jo951944n.
- ↑ S. Müller, B. Liepold, G. Roth and H. J. Bestmann* (1996). "An Improved One-pot Procedure for the Synthesis of Alkynes from Aldehydes". Synlett. 1996 (06): 521–522. doi:10.1055/s-1996-5474.
- ↑ G. Roth, B. Liepold, S. Müller and H. J. Bestmann (2004). "Further Improvements of the Synthesis of Alkynes from Aldehydes". Synthesis. 2004 (1): 59–62. doi:10.1055/s-2003-44346.
- ↑ Jepsen, T.H, Kristensen, J.L. J. Org. Chem. 2014, "In Situ Generation of the Ohira–Bestmann Reagent from Stable Sulfonyl Azide: Scalable Synthesis of Alkynes from Aldehydes". http://pubs.acs.org/doi/abs/10.1021/jo501803f
- ↑ Lidija Bondarenko; Ina Dix; Heino Hinrichs; Henning Hopf* (2004). "Cyclophanes. Part LII:1 Ethynyl[2.2]paracyclophanes – New Building Blocks for Molecular Scaffolding". Synthesis. 2004 (16): 2751–2759. doi:10.1055/s-2004-834872.