Sewage treatment
Sewage treatment is the process of removing contaminants from wastewater, primarily from household sewage. It includes physical, chemical, and biological processes to remove these contaminants and produce environmentally safe treated wastewater (or treated effluent). A by-product of sewage treatment is usually a semi-solid waste or slurry, called sewage sludge, that has to undergo further treatment before being suitable for disposal or land application.
Sewage treatment may also be referred to as wastewater treatment, although the latter is a broader term which can also be applied to purely industrial wastewater. For most cities, the sewer system will also carry a proportion of industrial effluent to the sewage treatment plant which has usually received pretreatment at the factories themselves to reduce the pollutant load. If the sewer system is a combined sewer then it will also carry urban runoff (stormwater) to the sewage treatment plant.
Terminology
The term "sewage treatment plant" (or "sewage treatment works" in some countries) is nowadays often replaced with the term "wastewater treatment plant".[1]
Sewage can be treated close to where the sewage is created, which may be called a "decentralized" system or even an "on-site" system (in septic tanks, biofilters or aerobic treatment systems). Alternatively, sewage can be collected and transported by a network of pipes and pump stations to a municipal treatment plant. This is called a "centralized" system (see also sewerage and pipes and infrastructure), although the borders between decentralized and centralized can be variable. For this reason, the terms "semi-decentralized" and "semi-centralized" are also being used.
Origins of sewage
Sewage is generated by residential, institutional, commercial and industrial establishments. It includes household waste liquid from toilets, baths, showers, kitchens, and sinks draining into sewers. In many areas, sewage also includes liquid waste from industry and commerce. The separation and draining of household waste into greywater and blackwater is becoming more common in the developed world, with treated greywater being permitted to be used for watering plants or recycled for flushing toilets.
Sewage mixing with rainwater
Sewage may include stormwater runoff or urban runoff. Sewerage systems capable of handling storm water are known as combined sewer systems. This design was common when urban sewerage systems were first developed, in the late 19th and early 20th centuries.[2]:119 Combined sewers require much larger and more expensive treatment facilities than sanitary sewers. Heavy volumes of storm runoff may overwhelm the sewage treatment system, causing a spill or overflow. Sanitary sewers are typically much smaller than combined sewers, and they are not designed to transport stormwater. Backups of raw sewage can occur if excessive infiltration/inflow (dilution by stormwater and/or groundwater) is allowed into a sanitary sewer system. Communities that have urbanized in the mid-20th century or later generally have built separate systems for sewage (sanitary sewers) and stormwater, because precipitation causes widely varying flows, reducing sewage treatment plant efficiency.[3]
As rainfall travels over roofs and the ground, it may pick up various contaminants including soil particles and other sediment, heavy metals, organic compounds, animal waste, and oil and grease. Some jurisdictions require stormwater to receive some level of treatment before being discharged directly into waterways. Examples of treatment processes used for stormwater include retention basins, wetlands, buried vaults with various kinds of media filters, and vortex separators (to remove coarse solids).[4]
Industrial effluent
In highly regulated developed countries, industrial effluent usually receives at least pretreatment if not full treatment at the factories themselves to reduce the pollutant load, before discharge to the sewer. This process is called industrial wastewater treatment. The same does not apply to many developing countries where industrial effluent is more likely to enter the sewer if it exists, or even the receiving water body, without pretreatment.
Industrial wastewater may contain pollutants which cannot be removed by conventional sewage treatment. Also, variable flow of industrial waste associated with production cycles may upset the population dynamics of biological treatment units, such as the activated sludge process.
Process steps
Overview
Sewage collection and treatment is typically subject to local, state and federal regulations and standards.
Treating wastewater has the aim to produce an effluent that will do as little harm as possible when discharged to the surrounding environment, thereby preventing pollution compared to releasing untreated wastewater into the environment.[5]
Sewage treatment generally involves three stages, called primary, secondary and tertiary treatment.
- Primary treatment consists of temporarily holding the sewage in a quiescent basin where heavy solids can settle to the bottom while oil, grease and lighter solids float to the surface. The settled and floating materials are removed and the remaining liquid may be discharged or subjected to secondary treatment. Some sewage treatment plants that are connected to a combined sewer system have a bypass arrangement after the primary treatment unit. This means that during very heavy rainfall events, the secondary and tertiary treatment systems can be bypassed to protect them from hydraulic overloading, and the mixture of sewage and stormwater only receives primary treatment.
- Secondary treatment removes dissolved and suspended biological matter. Secondary treatment is typically performed by indigenous, water-borne micro-organisms in a managed habitat. Secondary treatment may require a separation process to remove the micro-organisms from the treated water prior to discharge or tertiary treatment.
- Tertiary treatment is sometimes defined as anything more than primary and secondary treatment in order to allow rejection into a highly sensitive or fragile ecosystem (estuaries, low-flow rivers, coral reefs,...). Treated water is sometimes disinfected chemically or physically (for example, by lagoons and microfiltration) prior to discharge into a stream, river, bay, lagoon or wetland, or it can be used for the irrigation of a golf course, green way or park. If it is sufficiently clean, it can also be used for groundwater recharge or agricultural purposes.
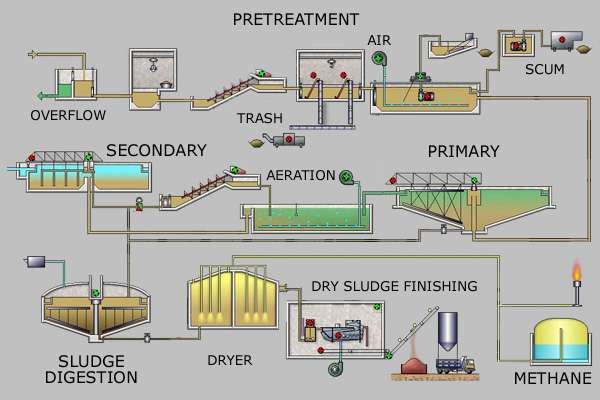
Pretreatment
Pretreatment removes all materials that can be easily collected from the raw sewage before they damage or clog the pumps and sewage lines of primary treatment clarifiers. Objects commonly removed during pretreatment include trash, tree limbs, leaves, branches, and other large objects.
The influent in sewage water passes through a bar screen to remove all large objects like cans, rags, sticks, plastic packets etc. carried in the sewage stream.[6] This is most commonly done with an automated mechanically raked bar screen in modern plants serving large populations, while in smaller or less modern plants, a manually cleaned screen may be used. The raking action of a mechanical bar screen is typically paced according to the accumulation on the bar screens and/or flow rate. The solids are collected and later disposed in a landfill, or incinerated. Bar screens or mesh screens of varying sizes may be used to optimize solids removal. If gross solids are not removed, they become entrained in pipes and moving parts of the treatment plant, and can cause substantial damage and inefficiency in the process.[7]:9
Grit removal
Pretreatment may include a sand or grit channel or chamber, where the velocity of the incoming sewage is adjusted to allow the settlement of sand, grit, stones, and broken glass. These particles are removed because they may damage pumps and other equipment. For small sanitary sewer systems, the grit chambers may not be necessary, but grit removal is desirable at larger plants.[7] Grit chambers come in 3 types: horizontal grit chambers, aerated grit chambers and vortex grit chambers. The process is called sedimentation.
Flow equalization
Clarifiers and mechanized secondary treatment are more efficient under uniform flow conditions. Equalization basins may be used for temporary storage of diurnal or wet-weather flow peaks. Basins provide a place to temporarily hold incoming sewage during plant maintenance and a means of diluting and distributing batch discharges of toxic or high-strength waste which might otherwise inhibit biological secondary treatment (including portable toilet waste, vehicle holding tanks, and septic tank pumpers). Flow equalization basins require variable discharge control, typically include provisions for bypass and cleaning, and may also include aerators. Cleaning may be easier if the basin is downstream of screening and grit removal.[8]
Fat and grease removal
In some larger plants, fat and grease are removed by passing the sewage through a small tank where skimmers collect the fat floating on the surface. Air blowers in the base of the tank may also be used to help recover the fat as a froth. Many plants, however, use primary clarifiers with mechanical surface skimmers for fat and grease removal.
Primary treatment

In the primary sedimentation stage, sewage flows through large tanks, commonly called "pre-settling basins", "primary sedimentation tanks" or "primary clarifiers".[9] The tanks are used to settle sludge while grease and oils rise to the surface and are skimmed off. Primary settling tanks are usually equipped with mechanically driven scrapers that continually drive the collected sludge towards a hopper in the base of the tank where it is pumped to sludge treatment facilities.[7]:9–11 Grease and oil from the floating material can sometimes be recovered for saponification (soap making).
Secondary treatment
Secondary treatment is designed to substantially degrade the biological content of the sewage which are derived from human waste, food waste, soaps and detergent. The majority of municipal plants treat the settled sewage liquor using aerobic biological processes. To be effective, the biota require both oxygen and food to live. The bacteria and protozoa consume biodegradable soluble organic contaminants (e.g. sugars, fats, organic short-chain carbon molecules, etc.) and bind much of the less soluble fractions into floc. Secondary treatment systems are classified as fixed-film or suspended-growth systems.
- Fixed-film or attached growth systems include trickling filters, bio-towers, and rotating biological contactors, where the biomass grows on media and the sewage passes over its surface.[7]:11–13 The fixed-film principle has further developed into Moving Bed Biofilm Reactors (MBBR)[10] and Integrated Fixed-Film Activated Sludge (IFAS) processes.[11] An MBBR system typically requires a smaller footprint than suspended-growth systems.[12]
- Suspended-growth systems include activated sludge, where the biomass is mixed with the sewage and can be operated in a smaller space than trickling filters that treat the same amount of water. However, fixed-film systems are more able to cope with drastic changes in the amount of biological material and can provide higher removal rates for organic material and suspended solids than suspended growth systems.[7]:11–13
Secondary sedimentation
Some secondary treatment methods include a secondary clarifier to settle out and separate biological floc or filter material grown in the secondary treatment bioreactor.
List of process types
To use less space, treat difficult waste, and intermittent flows, a number of designs of hybrid treatment plants have been produced. Such plants often combine at least two stages of the three main treatment stages into one combined stage. In the UK, where a large number of wastewater treatment plants serve small populations, package plants are a viable alternative to building a large structure for each process stage. In the US, package plants are typically used in rural areas, highway rest stops and trailer parks.[13]
Tertiary treatment
The purpose of tertiary treatment is to provide a final treatment stage to further improve the effluent quality before it is discharged to the receiving environment (sea, river, lake, wet lands, ground, etc.). More than one tertiary treatment process may be used at any treatment plant. If disinfection is practised, it is always the final process. It is also called "effluent polishing."
Filtration
Sand filtration removes much of the residual suspended matter.[7]:22–23 Filtration over activated carbon, also called carbon adsorption, removes residual toxins.[7]:19
Lagoons or ponds

Lagoons or ponds provide settlement and further biological improvement through storage in large man-made ponds or lagoons. These lagoons are highly aerobic and colonization by native macrophytes, especially reeds, is often encouraged. Small filter-feeding invertebrates such as Daphnia and species of Rotifera greatly assist in treatment by removing fine particulates.
Biological nutrient removal
Biological nutrient removal (BNR) is regarded by some as a type of secondary treatment process,[1] and by others as a tertiary (or "advanced") treatment process.
Wastewater may contain high levels of the nutrients nitrogen and phosphorus. Excessive release to the environment can lead to a buildup of nutrients, called eutrophication, which can in turn encourage the overgrowth of weeds, algae, and cyanobacteria (blue-green algae). This may cause an algal bloom, a rapid growth in the population of algae. The algae numbers are unsustainable and eventually most of them die. The decomposition of the algae by bacteria uses up so much of the oxygen in the water that most or all of the animals die, which creates more organic matter for the bacteria to decompose. In addition to causing deoxygenation, some algal species produce toxins that contaminate drinking water supplies. Different treatment processes are required to remove nitrogen and phosphorus.
Nitrogen removal
Nitrogen is removed through the biological oxidation of nitrogen from ammonia to nitrate (nitrification), followed by denitrification, the reduction of nitrate to nitrogen gas. Nitrogen gas is released to the atmosphere and thus removed from the water.
Nitrification itself is a two-step aerobic process, each step facilitated by a different type of bacteria. The oxidation of ammonia (NH3) to nitrite (NO2−) is most often facilitated by Nitrosomonas spp. ("nitroso" referring to the formation of a nitroso functional group). Nitrite oxidation to nitrate (NO3−), though traditionally believed to be facilitated by Nitrobacter spp. (nitro referring the formation of a nitro functional group), is now known to be facilitated in the environment almost exclusively by Nitrospira spp.
Denitrification requires anoxic conditions to encourage the appropriate biological communities to form. It is facilitated by a wide diversity of bacteria. Sand filters, lagooning and reed beds can all be used to reduce nitrogen, but the activated sludge process (if designed well) can do the job the most easily.[7]:17–18 Since denitrification is the reduction of nitrate to dinitrogen (molecular nitrogen) gas, an electron donor is needed. This can be, depending on the waste water, organic matter (from feces), sulfide, or an added donor like methanol. The sludge in the anoxic tanks (denitrification tanks) must be mixed well (mixture of recirculated mixed liquor, return activated sludge [RAS], and raw influent) e.g. by using submersible mixers in order to achieve the desired denitrification.
Sometimes the conversion of toxic ammonia to nitrate alone is referred to as tertiary treatment.
Over time, different treatment configurations have evolved as denitrification has become more sophisticated. An initial scheme, the Ludzack-Ettinger Process, placed an anoxic treatment zone before the aeration tank and clarifier, using the return activated sludge (RAS) from the clarifier as a nitrate source. Influent wastewater (either raw or as effluent from primary clarification) serves as the electron source for the facultative bacteria to metabolize carbon, using the inorganic nitrate as a source of oxygen instead of dissolved molecular oxygen. This denitrification scheme was naturally limited to the amount of soluble nitrate present in the RAS. Nitrate reduction was limited because RAS rate is limited by the performance of the clarifier.
The "Modified Ludzak-Ettinger Process" (MLE) is an improvement on the original concept, for it recycles mixed liquor from the discharge end of the aeration tank to the head of the anoxic tank to provide a consistent source of soluble nitrate for the facultative bacteria. In this instance, raw wastewater continues to provide the electron source, and sub-surface mixing maintains the bacteria in contact with both electron source and soluble nitrate in the absence of dissolved oxygen.
Many sewage treatment plants use centrifugal pumps to transfer the nitrified mixed liquor from the aeration zone to the anoxic zone for denitrification. These pumps are often referred to as Internal Mixed Liquor Recycle (IMLR) pumps. IMLR may be 200% to 400% the flow rate of influent wastewater (Q.) This is in addition to Return Activated Sludge (RAS) from secondary clarifiers, which may be 100% of Q. (Therefore, the hydraulic capacity of the tanks in such a system should handle at least 400% of annual average design flow (AADF.) At times, the raw or primary effluent wastewater must be carbon-supplemented by the addition of methanol, acetate, or simple food waste (molasses, whey, plant starch) to improve the treatment efficiency. These carbon additions should be accounted for in the design of a treatment facility's organic loading.[14]
Further modifications to the MLE were to come: Bardenpho and Biodenipho processes include additional anoxic and oxidative processes to further polish the conversion of nitrate ion to molecular nitrogen gas. Use of an anaerobic tank following the initial anoxic process allows for luxury uptake of phosphorus by bacteria, thereby biologically reducing orthophosphate ion in the treated wastewater. Even newer improvements, such as Anammox Process, interrupt the formation of nitrate at the nitrite stage of nitrification, shunting nitrite-rich mixed liquor activated sludge to treatment where nitrite is then converted to molecular nitrogen gas, saving energy, alkalinity, and secondary carbon sourcing. Anammox™ (ANaerobic AMMonia OXidation) works by artificially extending detention time and preserving denitrifiying bacteria through the use of substrate added to the mixed liquor and continuously recycled from it prior to secondary clarification. Many other proprietary schemes are being deployed, including DEMON™, Sharon-ANAMMOX™, ANITA-Mox™, and DeAmmon™.[15] The bacteria Brocadia anammoxidans can remove ammonium from waste water [16] through anaerobic oxidation of ammonium to hydrazine, a form of rocket fuel.[17][18]
Phosphorus removal
Every adult human excretes between 200 and 1000 grams of phosphorus annually. Studies of United States sewage in the late 1960s estimated mean per capita contributions of 500 grams in urine and feces, 1000 grams in synthetic detergents, and lesser variable amounts used as corrosion and scale control chemicals in water supplies.[19] Source control via alternative detergent formulations has subsequently reduced the largest contribution, but the content of urine and feces will remain unchanged. Phosphorus removal is important as it is a limiting nutrient for algae growth in many fresh water systems. (For a description of the negative effects of algae, see Nutrient removal). It is also particularly important for water reuse systems where high phosphorus concentrations may lead to fouling of downstream equipment such as reverse osmosis.
Phosphorus can be removed biologically in a process called enhanced biological phosphorus removal. In this process, specific bacteria, called polyphosphate-accumulating organisms (PAOs), are selectively enriched and accumulate large quantities of phosphorus within their cells (up to 20 percent of their mass). When the biomass enriched in these bacteria is separated from the treated water, these biosolids have a high fertilizer value.
Phosphorus removal can also be achieved by chemical precipitation, usually with salts of iron (e.g. ferric chloride), aluminum (e.g. alum), or lime.[7]:18 This may lead to excessive sludge production as hydroxides precipitates and the added chemicals can be expensive. Chemical phosphorus removal requires significantly smaller equipment footprint than biological removal, is easier to operate and is often more reliable than biological phosphorus removal. Another method for phosphorus removal is to use granular laterite.
Once removed, phosphorus, in the form of a phosphate-rich sewage sludge, may be dumped in a landfill or used as fertilizer. In the latter case, the treated sewage sludge is also sometimes referred to as biosolids.
Disinfection
The purpose of disinfection in the treatment of waste water is to substantially reduce the number of microorganisms in the water to be discharged back into the environment for the later use of drinking, bathing, irrigation, etc. The effectiveness of disinfection depends on the quality of the water being treated (e.g., cloudiness, pH, etc.), the type of disinfection being used, the disinfectant dosage (concentration and time), and other environmental variables. Cloudy water will be treated less successfully, since solid matter can shield organisms, especially from ultraviolet light or if contact times are low. Generally, short contact times, low doses and high flows all militate against effective disinfection. Common methods of disinfection include ozone, chlorine, ultraviolet light, or sodium hypochlorite.[7]:16 Chloramine, which is used for drinking water, is not used in the treatment of waste water because of its persistence. After multiple steps of disinfection, the treated water is ready to be released back into the water cycle by means of the nearest body of water or agriculture. Afterwards, the water can be transferred to reserves for everyday human uses.
Chlorination remains the most common form of waste water disinfection in North America due to its low cost and long-term history of effectiveness. One disadvantage is that chlorination of residual organic material can generate chlorinated-organic compounds that may be carcinogenic or harmful to the environment. Residual chlorine or chloramines may also be capable of chlorinating organic material in the natural aquatic environment. Further, because residual chlorine is toxic to aquatic species, the treated effluent must also be chemically dechlorinated, adding to the complexity and cost of treatment.
Ultraviolet (UV) light can be used instead of chlorine, iodine, or other chemicals. Because no chemicals are used, the treated water has no adverse effect on organisms that later consume it, as may be the case with other methods. UV radiation causes damage to the genetic structure of bacteria, viruses, and other pathogens, making them incapable of reproduction. The key disadvantages of UV disinfection are the need for frequent lamp maintenance and replacement and the need for a highly treated effluent to ensure that the target microorganisms are not shielded from the UV radiation (i.e., any solids present in the treated effluent may protect microorganisms from the UV light). In the United Kingdom, UV light is becoming the most common means of disinfection because of the concerns about the impacts of chlorine in chlorinating residual organics in the wastewater and in chlorinating organics in the receiving water. Some sewage treatment systems in Canada and the US also use UV light for their effluent water disinfection.[20][21]
Ozone (O3) is generated by passing oxygen (O2) through a high voltage potential resulting in a third oxygen atom becoming attached and forming O3. Ozone is very unstable and reactive and oxidizes most organic material it comes in contact with, thereby destroying many pathogenic microorganisms. Ozone is considered to be safer than chlorine because, unlike chlorine which has to be stored on site (highly poisonous in the event of an accidental release), ozone is generated on-site as needed. Ozonation also produces fewer disinfection by-products than chlorination. A disadvantage of ozone disinfection is the high cost of the ozone generation equipment and the requirements for special operators.
Fourth treatment stage
Micropollutants such as pharmaceuticals, ingredients of household chemicals, chemicals used in small businesses or industries, environmental persistent pharmaceutical pollutant (EPPP) or pesticides may not be eliminated in the conventional treatment process (primary, secondary and tertiary treatment) and therefore lead to water pollution.[22] Although concentrations of those substances and their decompostion products are quite low, there is still a chance to harm aquatic organisms. For pharmaceuticals, the following substances have been identified as "toxicologically relevant": substances with endocrine disrupting effects, genotoxic substances and substances that enhance the development of bacterial resistances.[23] They mainly belong to the group of environmental persistent pharmaceutical pollutants. Techniques for elimination of micropollutants via a fourth treatment stage during sewage treatment are being tested in Germany, Switzerland and the Netherlands.[24] However, since those techniques are still costly, they are not yet applied on a regular basis. Such process steps mainly consist of activated carbon filters that adsorb the micropollutants. Ozone can also be applied as an oxidative method.[25] Also the use of enzymes such as the enzyme laccase is under investigation.[26] A new concept which could provide an energy-efficient treatment of micropollutants could be the use of laccase secreting fungi cultivated at a wastewater treatment plant to degrade micropollutants and at the same time to provide enzymes at a cathode of a microbial biofuel cells.[27] Microbial biofuel cells are investigated for their property to treat organic matter in wastewater.[28]
To reduce pharmaceuticals in water bodies, also "source control" measures are under investigation, such as innovations in drug development or more responsible handling of drugs.[23][29]
Odor control
Odors emitted by sewage treatment are typically an indication of an anaerobic or "septic" condition.[30] Early stages of processing will tend to produce foul-smelling gases, with hydrogen sulfide being most common in generating complaints. Large process plants in urban areas will often treat the odors with carbon reactors, a contact media with bio-slimes, small doses of chlorine, or circulating fluids to biologically capture and metabolize the noxious gases.[31] Other methods of odor control exist, including addition of iron salts, hydrogen peroxide, calcium nitrate, etc. to manage hydrogen sulfide levels.
High-density solids pumps are suitable for reducing odors by conveying sludge through hermetic closed pipework.
Energy requirements
For conventional sewage treatment plants, around 30 percent of the annual operating costs is usually required for energy.[1]:1703 The energy requirements vary with type of treatment process as well as wastewater load. For example, constructed wetlands have a lower energy requirement than activated sludge plants, as less energy is required for the aeration step.[32] Sewage treatment plants that produce biogas in their sewage sludge treatment process with anaerobic digestion can produce enough energy to meet most of the energy needs of the sewage treatment plant itself.[1]:1505
In conventional secondary treatment processes, most of the electricity is used for aeration, pumping systems and equipment for the dewatering and drying of sewage sludge. Advanced wastewater treatment plants, e.g. for nutrient removal, require more energy than plants that only achieve primary or secondary treatment.[1]:1704
Sludge treatment and disposal
The sludges accumulated in a wastewater treatment process must be treated and disposed of in a safe and effective manner. The purpose of digestion is to reduce the amount of organic matter and the number of disease-causing microorganisms present in the solids. The most common treatment options include anaerobic digestion, aerobic digestion, and composting. Incineration is also used, albeit to a much lesser degree.[7]:19–21
Sludge treatment depends on the amount of solids generated and other site-specific conditions. Composting is most often applied to small-scale plants with aerobic digestion for mid-sized operations, and anaerobic digestion for the larger-scale operations.
The sludge is sometimes passed through a so-called pre-thickener which de-waters the sludge. Types of pre-thickeners include centrifugal sludge thickeners[33] rotary drum sludge thickeners and belt filter presses.[34][35][36] Dewatered sludge may be incinerated or transported offsite for disposal in a landfill or use as an agricultural soil amendment.
Environment aspects
Many processes in a wastewater treatment plant are designed to mimic the natural treatment processes that occur in the environment, whether that environment is a natural water body or the ground. If not overloaded, bacteria in the environment will consume organic contaminants, although this will reduce the levels of oxygen in the water and may significantly change the overall ecology of the receiving water. Native bacterial populations feed on the organic contaminants, and the numbers of disease-causing microorganisms are reduced by natural environmental conditions such as predation or exposure to ultraviolet radiation. Consequently, in cases where the receiving environment provides a high level of dilution, a high degree of wastewater treatment may not be required. However, recent evidence has demonstrated that very low levels of specific contaminants in wastewater, including hormones (from animal husbandry and residue from human hormonal contraception methods) and synthetic materials such as phthalates that mimic hormones in their action, can have an unpredictable adverse impact on the natural biota and potentially on humans if the water is re-used for drinking water.[37][38][39] In the US and EU, uncontrolled discharges of wastewater to the environment are not permitted under law, and strict water quality requirements are to be met, as clean drinking water is essential. (For requirements in the US, see Clean Water Act.) A significant threat in the coming decades will be the increasing uncontrolled discharges of wastewater within rapidly developing countries.
Effects on biology
Sewage treatment plants can have multiple effects on nutrient levels in the water that the treated sewage flows into. These nutrients can have large effects on the biological life in the water in contact with the effluent. Stabilization ponds (or sewage treatment ponds) can include any of the following:
- Oxidation ponds, which are aerobic bodies of water usually 1–2 meters in depth that receive effluent from sedimentation tanks or other forms of primary treatment.
- Dominated by algae
- Polishing ponds are similar to oxidation ponds but receive effluent from an oxidation pond or from a plant with an extended mechanical treatment.
- Dominated by zooplankton
- Facultative lagoons, raw sewage lagoons, or sewage lagoons are ponds where sewage is added with no primary treatment other than coarse screening. These ponds provide effective treatment when the surface remains aerobic; although anaerobic conditions may develop near the layer of settled sludge on the bottom of the pond.[2]:552–554
- Anaerobic lagoons are heavily loaded ponds.
- Dominated by bacteria
- Sludge lagoons are aerobic ponds, usually 2 to 5 meters in depth, that receive anaerobically digested primary sludge, or activated secondary sludge under water.
- Upper layers are dominated by algae [40]
Phosphorus limitation is a possible result from sewage treatment and results in flagellate-dominated plankton, particularly in summer and fall.[41]
A phytoplankton study found high nutrient concentrations linked to sewage effluents. High nutrient concentration leads to high chlorophyll a concentrations, which is a proxy for primary production in marine environments. High primary production means high phytoplankton populations and most likely high zooplankton populations, because zooplankton feed on phytoplankton. However, effluent released into marine systems also leads to greater population instability.[42]
The planktonic trends of high populations close to input of treated sewage is contrasted by the bacterial trend. In a study of Aeromonas spp. in increasing distance from a wastewater source, greater change in seasonal cycles was found the furthest from the effluent. This trend is so strong that the furthest location studied actually had an inversion of the Aeromonas spp. cycle in comparison to that of fecal coliforms. Since there is a main pattern in the cycles that occurred simultaneously at all stations it indicates seasonal factors (temperature, solar radiation, phytoplankton) control of the bacterial population. The effluent dominant species changes from Aeromonas caviae in winter to Aeromonas sobria in the spring and fall while the inflow dominant species is Aeromonas caviae, which is constant throughout the seasons.[43]
Treated sewage reuse
With suitable technology, it is possible to reuse sewage effluent for drinking water, although this is usually only done in places with limited water supplies, such as Windhoek and Singapore.[44]
In Israel, about 50 percent of agricultural water use (total use was 1 billion cubic metres in 2008) is provided through reclaimed sewer water. Future plans call for increased use of treated sewer water as well as more desalination plants.[45]
Sewage treatment in developing countries
Few reliable figures exist on the share of the wastewater collected in sewers that is being treated in the world. A global estimate by UNDP and UN-Habitat is that 90% of all wastewater generated is released into the environment untreated.[46] In many developing countries the bulk of domestic and industrial wastewater is discharged without any treatment or after primary treatment only.
In Latin America about 15 percent of collected wastewater passes through treatment plants (with varying levels of actual treatment). In Venezuela, a below average country in South America with respect to wastewater treatment, 97 percent of the country’s sewage is discharged raw into the environment.[47] In Iran, a relatively developed Middle Eastern country, the majority of Tehran's population has totally untreated sewage injected to the city’s groundwater.[48] However, the construction of major parts of the sewage system, collection and treatment, in Tehran is almost complete, and under development, due to be fully completed by the end of 2012. In Isfahan, Iran's third largest city, sewage treatment was started more than 100 years ago.
Only few cities in sub-Saharan Africa have sewer-based sanitation systems, let alone wastewater treatment plants, an exception being South Africa and – until the late 1990s- Zimbabwe.[49] Instead, most urban residents in sub-Saharan Africa rely on on-site sanitation systems without sewers, such as septic tanks and pit latrines, and faecal sludge management in these cities is an enormous challenge.[50]
History

Basic sewer systems were used for waste removal in ancient Mesopotamia, where vertical shafts carried the waste away into cesspools. Similar systems existed in the Indus Valley civilization in modern-day India and in Ancient Crete and Greece. In the Middle Ages the sewer systems built by the Romans fell into disuse and waste was collected into cesspools that were periodically emptied by workers known as 'rakers' who would often sell it as fertilizer to farmers outside the city.
Modern sewage systems were first built in the mid-nineteenth century as a reaction to the exacerbation of sanitary conditions brought on by heavy industrialization and urbanization. Due to the contaminated water supply, cholera outbreaks occurred in 1832, 1849 and 1855 in London, killing tens of thousands of people. This, combined with the Great Stink of 1858, when the smell of untreated human waste in the River Thames became overpowering, and the report into sanitation reform of the Royal Commissioner Edwin Chadwick,[51] led to the Metropolitan Commission of Sewers appointing Sir Joseph Bazalgette to construct a vast underground sewage system for the safe removal of waste. Contrary to Chadwick's recommendations, Bazalgette's system, and others later built in Continental Europe, did not pump the sewage onto farm land for use as fertilizer; it was simply piped to a natural waterway away from population centres, and pumped back into the environment.
Early attempts
One of the first attempts at diverting sewage for use as a fertilizer in the farm was made by the cotton mill owner James Smith in the 1840s. He experimented with a piped distribution system initially proposed by James Vetch[52] that collected sewage from his factory and pumped it into the outlying farms, and his success was enthusiastically followed by Edwin Chadwick and supported by organic chemist Justus von Liebig.
The idea was officially adopted by the Health of Towns Commission, and various schemes (known as sewage farms) were trialled by different municipalities over the next 50 years. At first, the heavier solids were channeled into ditches on the side of the farm and were covered over when full, but soon flat-bottomed tanks were employed as reservoirs for the sewage; the earliest patent was taken out by William Higgs in 1846 for "tanks or reservoirs in which the contents of sewers and drains from cities, towns and villages are to be collected and the solid animal or vegetable matters therein contained, solidified and dried..."[53] Improvements to the design of the tanks included the introduction of the horizontal-flow tank in the 1850s and the radial-flow tank in 1905. These tanks had to be manually de-sludged periodically, until the introduction of automatic mechanical de-sludgers in the early 1900s.[54]
The precursor to the modern septic tank was the cesspool in which the water was sealed off to prevent contamination and the solid waste was slowly liquified due to anaerobic action; it was invented by L.H Mouras in France in the 1860s. Donald Cameron, as City Surveyor for Exeter patented an improved version in 1895, which he called a 'septic tank'; septic having the meaning of 'bacterial'. These are still in worldwide use, especially in rural areas unconnected to large-scale sewage systems.[55]
Chemical treatment
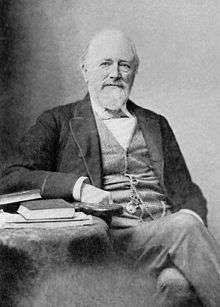
It was not until the late 19th century that it became possible to treat the sewage by chemically breaking it down through the use of microorganisms and removing the pollutants. Land treatment was also steadily becoming less feasible, as cities grew and the volume of sewage produced could no longer be absorbed by the farmland on the outskirts.
Sir Edward Frankland conducted experiments at the Sewage Farm in Croydon, England, during the 1870s and was able to demonstrate that filtration of sewage through porous gravel produced a nitrified effluent (the ammonia was converted into nitrate) and that the filter remained unclogged over long periods of time.[56] This established the then revolutionary possibility of biological treatment of sewage using a contact bed to oxidize the waste. This concept was taken up by the chief chemist for the London Metropolitan Board of Works, William Libdin, in 1887:
- ...in all probability the true way of purifying sewage...will be first to separate the sludge, and then turn into neutral effluent... retain it for a sufficient period, during which time it should be fully aerated, and finally discharge it into the stream in a purified condition. This is indeed what is aimed at and imperfectly accomplished on a sewage farm.[57]
From 1885 to 1891 filters working on this principle were constructed throughout the UK and the idea was also taken up in the US at the Lawrence Experiment Station in Massachusetts, where Frankland's work was confirmed. In 1890 the LES developed a 'trickling filter' that gave a much more reliable performance.[58]
Contact beds were developed in Salford, Lancashire and by scientists working for the London City Council in the early 1890s. According to Christopher Hamlin, this was part of a conceptual revolution that replaced the philosophy that saw "sewage purification as the prevention of decomposition with one that tried to facilitate the biological process that destroy sewage naturally."[59]
Contact beds were tanks containing the inert substance, such as stones or slate, that maximized the surface area available for the microbial growth to break down the sewage. The sewage was held in the tank until it was fully decomposed and it was then filtered out into the ground. This method quickly became widespread, especially in the UK, where it was used in Leicester, Sheffield, Manchester and Leeds. The bacterial bed was simultaneously developed by Joseph Corbett as Borough Engineer in Salford and experiments in 1905 showed that his method was superior in that greater volumes of sewage could be purified better for longer periods of time than could be achieved by the contact bed.[60]
The Royal Commission on Sewage Disposal published its eighth report in 1912 that set what became the international standard for sewage discharge into rivers; the '20:30 standard', which allowed 20 mg Biochemical oxygen demand and 30 mg suspended solid per litre.[61]
See also
References
- 1 2 3 4 5 Tchobanoglous, George; Burton, Franklin L.; Stensel, H. David; Metcalf & Eddy, Inc. (2003). Wastewater Engineering: Treatment and Reuse (4th ed.). McGraw-Hill. ISBN 0-07-112250-8.
- 1 2 Metcalf & Eddy, Inc. (1972). Wastewater Engineering. New York: McGraw-Hill. ISBN 0-07-041675-3.
- ↑ Burrian, Steven J., et al. (1999)."The Historical Development of Wet-Weather Flow Management." US Environmental Protection Agency (EPA). National Risk Management Research Laboratory, Cincinnati, OH. Document No. EPA/600/JA-99/275.
- ↑ Burton, Jr., G. Allen; Pitt, Robert E. (2001). "Chapter 2. Receiving Water Uses, Impairments, and Sources of Stormwater Pollutants". Stormwater Effects Handbook: A Toolbox for Watershed Managers, Scientists, and Engineers. New York: CRC/Lewis Publishers. ISBN 0-87371-924-7.
- ↑ Khopkar, S. M. (2004). Environmental Pollution Monitoring And Control. New Delhi: New Age International. p. 299. ISBN 81-224-1507-5.
- ↑ Water and Environmental Health at London and Loughborough (1999). "Waste water Treatment Options." Technical brief no. 64. London School of Hygiene & Tropical Medicine and Loughborough University.
- 1 2 3 4 5 6 7 8 9 10 11 EPA. Washington, DC (2004). "Primer for Municipal Waste water Treatment Systems." Document no. EPA 832-R-04-001.
- ↑ "Chapter 3. Flow Equalization". Process Design Manual for Upgrading Existing Wastewater Treatment Plants (Report). EPA. October 1971.
- ↑ Huber Company, Berching, Germany (2012). "Sedimentation Tanks."
- ↑ Barwal, Anjali; Chaudhary, Rubina (2014). "To study the performance of biocarriers in moving bed biofilm reactor (MBBR) technology and kinetics of biofilm for retrofitting the existing aerobic treatment systems: a review". Reviews in Environmental Science and Bio/Technology. Springer. 13 (3): 285–299. doi:10.1007/s11157-014-9333-7.
- ↑ Randall, Clifford W.; Sen, Dipankar (1996). "Full-scale evaluation of an integrated fixed-film activated sludge (IFAS) process for enhanced nitrogen removal". Water Science and Technology. Elsevier. 33 (12): 155–162. doi:10.1016/0273-1223(96)00469-6.
- ↑ "IFAS/MBBR Sustainable Wastewater Treatment Solutions" (PDF). Black & Veatch, Inc. 2009. Archived from the original (PDF) on 2010-12-14. Brochure.
- ↑ EPA. Washington, DC (2000). "Package Plants." Wastewater Technology Fact Sheet. Document no. EPA 832-F-00-016.
- ↑ http://www.wefnet.org/mopnew/Operation_of_Municipal_Wastewater_Treatment_Plants/Chapter%2022%20Revised_6th%20Edition.pdf
- ↑ "Chapter 3. Biological Treatment Processes". Emerging Technologies for Wastewater Treatment and In-Plant Wet Weather Management (Report). EPA. March 2013. EPA 832-R-12-011.
- ↑ B. Kartal, G.J. Kuenen and M.C.M van Loosdrecht, Sewage Treatment with Anammox, Science, 2010, vol 328 p 702-3
- ↑ http://news.nationalgeographic.com/news/2005/11/1109_051109_rocketfuel.html
- ↑ Harhangi, H. R.; Le Roy, M; Van Alen, T; Hu, B. L.; Groen, J; Kartal, B; Tringe, S. G.; Quan, Z. X.; Jetten, M. S.; Op Den Camp, H. J. (2012). "Hydrazine synthase, a unique phylomarker with which to study the presence and biodiversity of anammox bacteria". Appl. Environ. Microbiol. 78 (3): 752–8. doi:10.1128/AEM.07113-11. PMC 3264106
 . PMID 22138989.
. PMID 22138989. - ↑ Process Design Manual for Phosphorus Removal (Report). EPA. 1976. pp. 2–1. EPA 625/1-76-001a.
- ↑ Das, Tapas K. (August 2001). "Ultraviolet disinfection application to a wastewater treatment plant". Clean Technologies and Environmental Policy. Springer Berlin/Heidelberg. 3 (2): 69–80. doi:10.1007/S100980100108.
- ↑ Florida Department of Environmental Protection. Tallahassee, FL. "Ultraviolet Disinfection for Domestic Waste water." 2010-03-17.
- ↑ UBA (Umweltbundesamt) (2014): Maßnahmen zur Verminderung des Eintrages von Mikroschadstoffen in die Gewässer. Texte 85/2014
- 1 2 Walz, A., Götz, K. (2014): Arzneimittelwirkstoffe im Wasserkreislauf. ISOE-Materialien zur Sozialen Ökologie Nr. 36 (in German)
- ↑ Hernández Leal, L. (2010). Removal of micropollutants from grey water – Combining biological and physical/chemical processes. PhD thesis, Wageningen University
- ↑ Ternes, T. (2009): Challenges for the future: emerging micropollutants in urban water cycle. BFG, Koblenz, Germany (presentation for a seminar at UNESCO-IHE, Delft)
- ↑ Margot, J.; et al. (2013). "Bacterial versus fungal laccase: potential for micropollutant degradation". AMB Express. 3: 63. doi:10.1186/2191-0855-3-63.
- ↑ Crude mushroom solution to degrade micropollutants and increase the performance of biofuel cells: https://www.biooekonomie-bw.de/en/articles/news/crude-mushroom-solution-to-degrade-micropollutants-and-increase-the-performance-of-biofuel-cells/
- ↑ Logan, B.; Regan, J. (2006). "Microbial Fuel Cells—Challenges and Applications". Environmental Science & Technology. 40 (17): 5172–5180. doi:10.1021/es0627592.
- ↑ Lienert, J.; Bürki, T.; Escher, B.I. (2007). "Reducing micropollutants with source control: Substance flow analysis of 212 pharmaceuticals in faeces and urine". Water Science & Technology. 56 (5): 87–96. doi:10.2166/wst.2007.560.
- ↑ Harshman, Vaughan; Barnette, Tony (2000-12-28). "Wastewater Odor Control: An Evaluation of Technologies". Water Engineering & Management. ISSN 0273-2238.
- ↑ Walker, James D. and Welles Products Corporation (1976)."Tower for removing odors from gases." U.S. Patent No. 4421534.
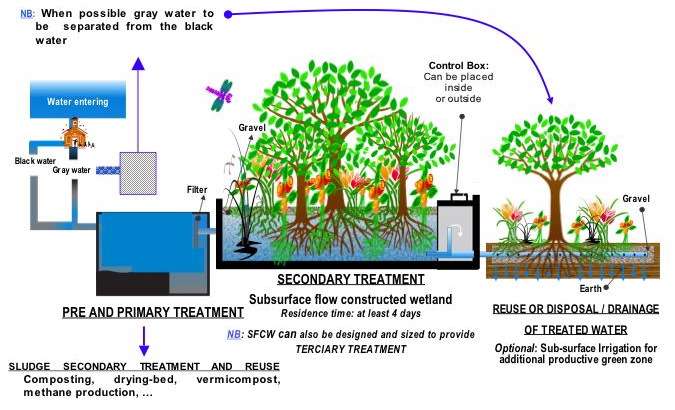
- ↑ Hoffmann, H., Platzer, C., von Münch, E., Winker, M. (2011). Technology review of constructed wetlands – Subsurface flow constructed wetlands for greywater and domestic wastewater treatment. Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH, Eschborn, Germany, p. 11
- ↑ IPEC Consultants, Ltd., Burnaby, BC, Canada. "IFT – Internally-fed – rotary thickener." Accessed 2012-06-16.
- ↑ Mine-engineer.com, Long Beach, CA. "Belt Filter Press, How Does It Work?" Accessed 2012-06-16.
- ↑ GlobalSpec, Inc., East Greenbush, NY and Eco tubes.
- ↑ "How to Select Dewatering Equipment." Accessed 2012-06-12.
- ↑ Environment Agency (archive) – Persistent, bioaccumulative and toxic PBT substances at the Wayback Machine (archived August 4, 2006). environment-agency.gov.uk. Retrieved on 2012-12-19.
- ↑ Natural Environmental Research Council – River sewage pollution found to be disrupting fish hormones. Planetearth.nerc.ac.uk. Retrieved on 2012-12-19.
- ↑ Endocrine Disruption Found in Fish Exposed to Municipal Wastewater at the Wayback Machine (archived October 15, 2011). USGS
- ↑ Haughey, A. (1968). "The Planktonic Algae of Auckland Sewage Treatment Ponds". New Zealand Journal of Marine and Freshwater Research. 2 (4): 721–766. doi:10.1080/00288330.1968.9515271.
- ↑ Edmondson, W.T. (1972). "Nutrients and Phytoplankton in Lake Washington." in Nutrients and Eutrophication: The Limiting Nutrient Controversy. American Society of Limnology and Oceanography, Special Symposia. Vol. 1.
- ↑ Caperon, J.; Cattell, S. A. & Krasnick, G. (1971). "Phytoplankton Kinetics in a Subtropical Estuary: Eutrophication" (PDF). Limnology and Oceanography. 16 (4): 599–607. doi:10.4319/lo.1971.16.4.0599.
- ↑ Monfort, P; Baleux, B (1990). "Dynamics of Aeromonas hydrophila, Aeromonas sobria, and Aeromonas caviae in a sewage treatment pond". Applied and Environmental Microbiology. 56 (7): 1999–2006. PMC 184551
 . PMID 2389929.
. PMID 2389929. - ↑ PUB (Singapore National Water Agency)(2011). "NEWater: History."
- ↑ Martin, Andrew (2008-08-10). "Farming in Israel, without a drop to spare". New York Times.
- ↑ Corcoran, E., C. Nellemann, E. Baker, R. Bos, D. Osborn, H. Savelli (eds) (2010). Sick water? : the central role of wastewater management in sustainable development : a rapid response assessment (PDF). Arendal, Norway: UNEP/GRID-Arendal. ISBN 978-82-7701-075-5.
- ↑ Caribbean Environment Programme (1998). Appropriate Technology for Sewage Pollution Control in the Wider Caribbean Region (PDF). Kingston, Jamaica: United Nations Environment Programme. Retrieved 2009-10-12. Technical Report No. 40.
- ↑ Massoud Tajrishy and Ahmad Abrishamchi (2005). "Integrated Approach to Water and Wastewater Management for Tehran, Iran." Water Conservation, Reuse, and Recycling: Proceedings of the Iranian-American Workshop. Washington, D.C.: National Academies Press.
- ↑ Zimbabwe: Water and Sanitation Crisis, Human Rights Watch, https://www.hrw.org/news/2013/11/19/zimbabwe-water-and-sanitation-crisis
- ↑ Chowdhry, S., Koné, D. (2012). Business Analysis of Fecal Sludge Management: Emptying and Transportation Services in Africa and Asia – Draft final report. Bill & Melinda Gates Foundation, Seattle, USA
- ↑ Ashton, John; Ubido, Janet (1991). "The Healthy City and the Ecological Idea" (PDF). Journal of the Society for the Social History of Medicine. 4 (1): 173–181. doi:10.1093/shm/4.1.173. Retrieved 8 July 2013.
- ↑ Lewis Dunbar B. Gordon (1851). A short description of the plans of Captain James Vetch for the sewerage of the metropolis.
- ↑ H. H. Stanbridge (1976). History of Sewage Treatment in Britain. Institute of Water Pollution Control.
- ↑ P. F. Cooper. "Historical aspects of wastewater treatment" (PDF). Retrieved 2013-12-21.
- ↑ Martin V. Melosi (2010). The Sanitary City: Environmental Services in Urban America from Colonial Times to the Present. University of Pittsburgh Press. p. 110. ISBN 9780822973379.
- ↑ Colin A. Russell (2003). Edward Frankland: Chemistry, Controversy and Conspiracy in Victorian England. Cambridge University Press. pp. 372–380. ISBN 9780521545815.
- ↑ Sharma, Sanjay Kumar; Sanghi, Rashmi (2012). Advances in Water Treatment and Pollution Prevention. Springer. ISBN 9789400742048. Retrieved 2013-02-07.
- ↑ Epidemics, demonstration effects, and municipal investment in sanitation capital.
- ↑ "Edwin Chadwick and the Engineers, 1842–1854: Systems and Antisystems in the Pipe-and-Brick Sewers War Technology and Culture" (PDF). 1992.
- ↑ Tilley, David F. (2011). Aerobic Wastewater Treatment Processes: History and Development. IWA Publishing. ISBN 9781843395423. Retrieved 2013-02-07.
- ↑ Final report of the commissioners appointed to inquire and report what methods of treating and disposing of sewage (1912). us.archive.org
External links
| Wikimedia Commons has media related to Wastewater treatment plants. |
- Interactive Diagram of Wastewater Treatment – "Go with the Flow" – Water Environment Federation, (available in English and Spanish and French).
- Improved Modelling of Wastewater Treatment Primary Clarifier Using Hybrid Anns


