Protonic ceramic fuel cell
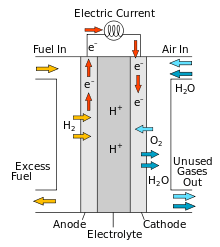
A protonic ceramic fuel cell or PCFC is a fuel cell based on a ceramic electrolyte material that exhibits high protonic conductivity at elevated temperatures.
PCFCs share the thermal and kinetic advantages of high temperature operation at 700 degrees Celsius with molten carbonate and solid oxide fuel cells, while exhibiting all of the intrinsic benefits of proton conduction in proton exchange membrane fuel cells (PEMFC) and phosphoric acid fuel cells (PAFC). The high operating temperature is necessary to achieve very high electrical fuel efficiency with hydrocarbon fuels. PCFCs can operate at high temperatures and electrochemically oxidize fossil fuels directly to the anode. This eliminates the intermediate step of producing hydrogen through the costly reforming process. Gaseous molecules of the hydrocarbon fuel are absorbed on the surface of the anode in the presence of water vapor, and hydrogen atoms are efficiently stripped off to be absorbed into the electrolyte, with carbon dioxide as the primary reaction product. PCFCs have a solid electrolyte, so that the membrane cannot dry out as with PEM fuel cells, and liquid cannot leak out as with PAFCs.
CoorsTek is primarily researching this type of fuel cell.