New Zealand mud snail
| New Zealand mudsnail | |
|---|---|
 | |
| right side view of Potamopyrgus antipodarum | |
 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Mollusca |
| Class: | Gastropoda |
| (unranked): | clade Caenogastropoda clade Hypsogastropoda |
| Superfamily: | Rissooidea |
| Family: | Hydrobiidae |
| Genus: | Potamopyrgus |
| Species: | P. antipodarum |
| Binomial name | |
| Potamopyrgus antipodarum J. E. Gray, 1843[2] | |
| Synonyms | |
| |
The New Zealand mudsnail (Potamopyrgus antipodarum) sometimes previously known as Potamopyrgus jenkinsi, is a species of very small or minute freshwater snail with a gill and an operculum, an aquatic gastropod mollusk in the family Hydrobiidae.
It is an invasive species in many countries, where populations of the snail can reach phenomenal densities.
Forms
- Potamopyrgus antipodarum f. carinata (J. T. Marshall, 1889)
Shell description

and Potamopyrgus antipodarum (right).
Scale bar is 0.5 cm.

The shell of Potamopyrgus antipodarum is elongated and has dextral coiling. The shell has 7 or 8 whorls. Between whorls are deep grooves.
It is an operculate snail, with a 'lid' that can seal the opening of its shell. The operculum is thin and corneus with an off-centre nucleus from which paucispiral markings (with few coils) radiate. The aperture is oval and its height is less than the height of the spire.
Some morphs, including many from the Great Lakes, exhibit a keel in the middle of each whorl; others, excluding those from the Great Lakes, exhibit periostracal ornamentation such as spines for anti–predator defense.[3][4][5][6]
Shell colors vary from gray and dark brown to light brown.
The average height of the shell is approximately 5 mm ( in); maximum size is approximately 12 mm ( in). The snail is usually 4–6 mm in length in the Great Lakes, but grows to 12 mm in its native range.[3][5][6]
Taxonomy
This species was originally described as Amnicola antipodarum in 1843 by John Edward Gray:
Inhabits New Zealand, in fresh water. Shell ovate, acute, subperforated (generally covered with a brown earthy coat); whorls rather rounded, mouth ovate, axis 3 lines; operculum horny and subspiral: variety, spire rather longer, whorls more rounded. This species is like Paludina nigra of Quoy and Gaimard, but the operculum is more spiral. Quoy described the operculum as concentric, but figured it subspiral. Paludina ventricosa of Quoy is evidently a Nematura.[2]
Distribution
This species is endemic to New Zealand. It lives in freshwater streams and lakes in New Zealand and adjacent small islands.[7]
Nonindigenous distribution
While endemic to New Zealand, the New Zealand mudsnail has spread widely and has become naturalised and an invasive species in many areas including: Australia, Tasmania, Asia (Japan,[8] in Garmat Ali River in Iraq since 2008[9]), Europe (since 1859 in England), and North America (USA and Canada: Thunder Bay in Ontario since 2001, British Columbia since July 2007[8]), most likely due to inadvertent human intervention.
Invasion in Europe
- List of non-marine molluscs of Ireland around 1837 Stelfox, A. W. (1926) [10] - probably the first introduction in Europe
- England since 1859 [11]
- Germany
- Poland
- Western Baltic Sea since 1887[12]
- Russia
- Azov Black Sea region, since 1951,[13] Ukraine since 1951 in brackish waters, and since 2005 in freshwater[14]
- Catalonia in Spain, since 1952[15]
- Mediterranean region of France, since the end of 1950s[15]
- Italy, since 1961[15]
- Turkey[15]
- Czech Republic, since September 3, 1981[16]
- Slovakia, since 1986[11]
- Greece, since November 2007[15]
- and other areas
Potamopyrgus antipodarum occurs in nearly the whole of Europe. It does not occur in Iceland, Albania, Bulgaria or the former Yugoslavia.[13]
Distribution within the USA
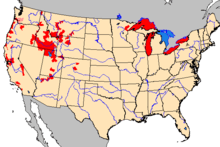
First detected in the United States in Idaho's Snake River in 1987, the mudsnail has since spread to the Madison River, Firehole River, and other watercourses around Yellowstone National Park; samples have been discovered throughout the western United States.[6] Although the exact means of transmission is unknown, it is likely that it was introduced in water transferred with live game fish and has been spread by ship ballast or contaminated recreational equipment such as wading gear.[17]
The New Zealand mudsnail has no natural predators or parasites in the United States, and consequently has become an invasive species. Densities have reached greater than 300,000 individuals per m² in the Madison River. It can reach concentrations greater than 500,000 per m², endangering the food chain by outcompeting native snails and water insects for food, leading to sharp declines in native populations.[18] Fish populations then suffer because the native snails and insects are their main food source.
Mudsnails are impressively resilient. A snail can live for 24 hours without water. They can however survive for up to 50 days on a damp surface,[19] giving them ample time to be transferred from one body of water to another on fishing gear. The snails may even survive passing through the digestive systems of fish and birds.
Mudsnails have now spread from Idaho to most western states of the U.S., including Wyoming, California, Nevada, Oregon, Montana, and Colorado. Environmental officials for these states have attempted to slow the spread of the snail by advising the public to keep an eye out for the snails, and bleach or heat any gear which may contain mudsnails. Rivers have also been temporarily closed to fishing to avoid anglers spreading the snails.[20][21]
The snails grow to a smaller size in the U.S. than in their native habitat, reaching 6 mm (¼ in) at most in parts of Idaho, but can be much smaller making them easy to overlook when cleaning fishing gear.
Clonal species like the New Zealand mudsnail can often develop clonal lines with quite diverse appearances, called morphs. Until 2005, all the snails found in the western states of the U.S. were believed to be from a single line. However a second morph has been identified in Idaho's Snake River. It grows to a similar size but has a distinctive appearance. (It has been nicknamed the salt-and-pepper mudsnail due to the final whorl being lighter than the rest of the shell.) This morph has apparently been present in the area for several years before being identified correctly as a distinct morph of Potamopyrgus antipodarum. It dominates the typical morph where they overlap, and has a much higher prevalence of males.[22]
In 1991 the New Zealand mudsnail was discovered in Lake Ontario,[23] and has now been found in four of the five Great Lakes. In 2005 and 2006, it was found to be widespread in Lake Erie.[24] By 2006 it had spread to Duluth-Superior Harbour and the freshwater estuary of the Saint Louis River.[25] It was found to be inhabiting Lake Michigan, after scientists took water samples in early summer of 2008.[26] The snails in the Great Lakes represent a different line from those found in western states, and were probably introduced indirectly through Europe.[22]
In 2009, the species was discovered in Capitol Lake in Olympia, Washington. The lake has been closed to all public use, including boating and other recreation, since 2009.[27] A heavy cold snap in 2013, combined with a drawdown in water level in preparation, was roughly estimated to have killed 40–60% of the mudsnail population.[28][29]
In 2010, the Los Angeles Times reported that the New Zealand mudsnail had infested watersheds in the Santa Monica Mountains, posing serious threats to native species and complicating efforts to improve stream-water quality for the endangered Southern California Distinct Population Segment of steelhead.[30] According to the article, the snails have expanded "from the first confirmed sample in Medea Creek in Agoura Hills to nearly 30 other stream sites in four years." Researchers at the Santa Monica Bay Restoration Commission believe that the snails' expansion may have been expedited after the mollusks traveled from stream to stream on the gear of contractors and volunteers.[31]
As of 21 September 2010 In Colorado, Boulder Creek and Dry Creek have infestations of New Zealand mudsnails. The snails have been present in Boulder Creek since 2004 and were discovered in Dry Creek in September 2010. Access to both creeks has been closed to help avoid spread of the snails. In the summer of 2015 an industrial-scale wetland rehabilitation project was undertaken in northeast Boulder to rid the area of a mud snail infestation.
Ecology
Habitat
The snail tolerates siltation, thrives in disturbed watersheds, and benefits from high nutrient flows allowing for filamentous green algae growth. It occurs amongst macrophytes and prefers littoral zones in lakes or slow streams with silt and organic matter substrates, but tolerates high flow environments where it can burrow into the sediment.[3][6][32][33][34][35][36][37][38][39][40]
In the Great Lakes, the snail reaches densities as high as 5,600 per m² and is found at depths of 4–45 m on a silt and sand substrate.[3][5][6]
This species is euryhaline, establishing populations in fresh and brackish water. The optimal salinity is probably near or below 5 ppt, but Potamopyrgus antipodarum is capable of feeding, growing, and reproducing at salinities of 0–15 ppt and can tolerate 30–35 ppt for short periods of time.[3][6][41][42][43][44]
It tolerates temperatures of 0–34 °C.[3][6][45]
Feeding habits
Potamopyrgus antipodarum is a nocturnal grazer-scraper, feeding on plant and animal detritus, epiphytic and periphytic algae, sediments and diatoms.[3][6][46][47][48][49]
Life cycle
Potamopyrgus antipodarum is ovoviviparous and parthenogenic. This means that they can reproduce asexually; females "are born with developing embryos in their reproductive system." Native populations in New Zealand consist of diploid sexual and triploid parthenogenically cloned females, as well as sexually functional males (less than 5% of the total population). All introduced populations in North America are clonal, consisting of genetically identical females.[6]
As the snails can reproduce both sexually and asexually, the snail has been used as a model organism for studying the costs and benefits of sexual reproduction. Asexual reproduction allows all members of a population to produce offspring and avoids the costs involved in finding mates. However, asexual offspring are clonal, so lack variation. This makes them susceptible to parasites, as the entire clonal population has the same resistance mechanisms. Once a strain of parasite has overcome these mechanisms, it is able to infect any member of the population. Sexual reproduction mixes up resistance genes through crossing over and the random assortment of gametes in meiosis, meaning the members of a sexual population will all have subtly different combinations of resistance genes. This variation in resistance genes means no one parasite strain is able to sweep through the whole population. New Zealand mudsnails are commonly infected with trematode parasites, which are particularly abundant in shallow water, but scarce in deeper water. As predicted, sexual reproduction dominates in shallow water, due to its advantages in parasite resistance. Asexual reproduction is dominant in the deeper water of lakes, as the scarcity of parasites means that the advantages of resistance are outweighed by the costs of sexual reproduction.[50]
Each female can produce between 20 and 120 embryos.[17] The snail produces approximately 230 young per year. Reproduction occurs in spring and summer, and the life cycle is annual.[3][6][7][44][51][52] The rapid reproduction rate of the snail has caused the numbers of individuals to increase rapidly in new environments. The highest concentration of New Zealand mudsnails ever reported was in Lake Zurich, Switzerland, where the species colonized the entire lake within seven years to a density of 800,000 per m².[6][53]
Parasites
In their native habitat, the snails pose no problem because of a trematode parasite which sterilizes many snails, keeping the populations to a manageable size. However they have become an invasive pest species elsewhere in the world in the absence of these parasites.[6]
The parasites of this species include at least 11 species of Trematoda.[6][54]
Common parasites of this snail include trematodes of the genus Microphallus.[6][55][56]
Other interspecific relationship
Potamopyrgus antipodarum can survive passage through the guts of fish and birds and may be transported by these animals.[57]
It can also float by itself or on mats of Cladophora spp., and move 60 m upstream in 3 months through positive rheotactic behavior.[3] It can respond to chemical stimuli in the water, including the odor of predatory fish, which causes it to migrate to the undersides of rocks to avoid predation.[6][58]
See also
References
- ↑ Van Damme, D. (2013). "Potamopyrgus antipodarum". IUCN Red List of Threatened Species. Version 2013.2. International Union for Conservation of Nature. Retrieved 20 February 2014.
- 1 2 Dieffenbach, E. 1843. Travels in New Zealand; with contributions to the geography, geology, botany, and natural history of that country. In two volumes - Vol. II. - pp. i-iv [= 1-4], 1-396, pl. [1]. London. (Murray), page 241.
- 1 2 3 4 5 6 7 8 9 Zaranko, D. T., D. G. Farara and F. G. Thompson. 1997. Another exotic mollusk in the Laurentian Great Lakes: the New Zealand native Potamopyrgus antipodarum (Gray 1843) (Gastropoda, Hydrobiidae).
- ↑ Holomuzki, J. R. and B. J. F. Biggs. 2006. Habitat–specific variation and performance trade–offs in shell armature of New Zealand mudsnails. Ecology 87(4):1038–1047.
- 1 2 3 Levri, E.P., A.A. Kelly and E. Love. 2007. The invasive New Zealand mud snail (Potamopyrgus antipodarum) in Lake Erie. Journal of Great Lakes Research 33: 1–6.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Benson, A.J.; Kipp, R.M.; Larson, J. & Fusaro, A. (2013). "Potamopyrgus antipodarum". USGS Nonindigenous Aquatic Species Database. U.S. Geological Survey. Retrieved 25 May 2013.
- 1 2 Hall, R. O. Jr., J. L. Tank and M. F. Dybdahl. 2003. Exotic snails dominate nitrogen and carbon cycling in a highly productive stream. Frontiers in Ecology and the Environment 1(8):407–411.
- 1 2 Timothy M. Davidson, Valance E. F. Brenneis, Catherine de Rivera, Robyn Draheim & Graham E. Gillespie. Northern range expansion and coastal occurrences of the New Zealand mud snail Potamopyrgus antipodarum (Gray, 1843) in the northeast Pacific Aquatic Invasions (2008) Volume 3, Issue 3: 349-353.
- ↑ Murtada D. Naser & Mikhail O. Son. 2009. First record of the New Zealand mud snail Potamopyrgus antipodarum (Gray 1843) from Iraq: the start of expansion to Western Asia?. Aquatic Invasions, Volume 4, Issue 2: 369-372, DOI 10.3391/ai.2009.4.2.11.
- ↑ Paludestrina jenkinsi in Lough Neagh and elsewhere. Irish Naturalists’ Journal 1: 174-175.
- 1 2 Čejka T., Dvořák L. & Košel V. 2008: Present distribution of Potamopyrgus antipodarum (Gray, 1843) (Mollusca: Gastropoda) in the Slovak Republic. - Malacologica Bohemoslovaca, 7: 21-25. Online serial at <http://mollusca.sav.sk> 25-February-2008.
- ↑ Dmitry P. Filippenko & Mikhail O. Son. The New Zealand mud snail Potamopyrgus antipodarum (Gray, 1843) is colonising the artificial lakes of Kaliningrad City, Russia (Baltic Sea Coast). Aquatic Invasions (2008) Volume 3, Issue 3: 345-347.
- 1 2 Mikhail O. Son. Rapid expansion of the New Zealand mud snail Potamopyrgus antipodarum (Gray, 1843) in the Azov-Black Sea Region. Aquatic Invasions (2008) Volume 3, Issue 3: 335-340.
- ↑ Boris Alexandrov, Alexandr Boltachev, Taras Kharchenko, Artiom Lyashenko, Mikhail Son, Piotr Tsarenko & Valeriy Zhukinsky. Trends of aquatic alien species invasions in Ukraine. Aquatic Invasions (2007) Volume 2, Issue 3: 215-242.
- 1 2 3 4 5 Canella Radea, Ioanna Louvrou and Athena Economou-Amilli First record of the New Zealand mud snail Potamopyrgus antipodarum J.E. Gray 1843 (Mollusca: Hydrobiidae) in Greece – Notes on its population structure and associated microalgae. Aquatic Invasions (2008) Volume 3, Issue 3: 341-344
- ↑ Kuchař P. Potamopyrgus jenkinsi poprvé v Československu. Źiva, Prague, 31(1): page 23. (in Czech).
- 1 2 "Biology". New Zealand mudsnails in the Western USA. Montana State University. Retrieved 2006-05-04.
- ↑ Benson, Amy (2006). "New Zealand Mudsnail: Potamopyrgus antipodarum". Florida Integrated Science Center. Retrieved 2006-05-04.
- ↑ Davis, Ken W. (2004-02-24). "Select Research Findings on the New Zealand Mudsnail (Potamopyrgus antipodarum)" (PDF). Wildlife Survey & Photo Service. p. 1. Retrieved 2006-05-07.
- ↑ "Non-native snail turns up in Truckee River". Elko Daily Free Press. 20 May 2013. p. 4.
- ↑ "News Release - Discovery of Invasive New Zealand Mud Snail Forces Temporary Closure of Putah Creek". California Department of Fish and Game. 2003-12-16. Retrieved 2006-05-07.
- 1 2 "Western USA Potamopyrgus antipodarum morphs". Department of Ecology, Montana State University-Bozeman. 2006-02-22. Retrieved 2006-05-07.
- ↑ Levri, E. P., Dermott, R. M., Lunnen, S. J., Kelly, A. A., and Ladson, T. (2008). The distribution of the invasive New Zealand mud snail (Potamopyrgus antipodarm) in Lake Ontario, Aquatic Ecosystem Health and Management 11(4), 412-421.
- ↑ New Zealand Mud Snails Invade Lake Erie!, International Association for Great Lakes Research, 26 March 2007.
- ↑ "Invasive snail found in Minn. harbor". Associated Press. May 3, 2006.
- ↑ "Worrying invasive snail found in Lake Michigan". The Associated Press. 2008-08-16.
- ↑ Dodge, John (19 October 2010). "Snail seclusion successful". The Olympian.
- ↑ Shannon, Brad (4 December 2013). "Freeze could help kill Capitol Lake's mudsnail population". The News Tribune.
- ↑ Shannon, Brad (26 December 2013). "Cold estimated to have killed half of snails in Capitol Lake". The Olympian.
- ↑ "South-Central/Southern California Coast Steelhead Recovery Planning Domain 5-Year Review: Summary and Evaluation of Southern California Coast Steelhead Distinct Population Segment" (PDF). National Oceanic and Atmospheric Administration. 2011. Retrieved 2013-12-03.
- ↑ Hard-to-kill snails infest Santa Monica Mountain watersheds Even Formula 409 has proven ineffective at destroying the New Zealand mudsnail, an asexually reproducing invasive species that poses a threat to steelhead restoration efforts and native creatures.
- ↑ Collier, K. J., R. J. Wilcock and A. S. Meredith. 1998. Influence of substrate type and physico–chemical conditions on macroinvertebrate faunas and biotic indices in some lowland Waikato, New Zealand, streams. New Zealand Journal of Marine and Freshwater Research 32(1):1–19.
- ↑ Holomuzki, J. R. and B. J. F. Biggs. 1999. Distributional responses to flow disturbance by a stream–dwelling snail. Oikos 87(1):36–47.
- ↑ Holomuzki, J. R. and B. J. F. Biggs. 2000. Taxon–specific responses to high–flow disturbances in streams: implications for population persistence. Journal of the North American Benthological Society 19(4):670–679.
- ↑ Negovetic, S. and J. Jokela. 2000. Food choice behaviour may promote habitat specificity in mixed populations of clonal and sexual Potamopyrgus antipodarum. Experimental Ecology 60(4):435–441.
- ↑ Richards, D. C., L. D. Cazier and G. T. Lester. 2001. Spatial distribution of three snail species, including the invader Potamopyrgus antipodarum, in a freshwater spring. Western North American Naturalist 61(3):375–380.
- ↑ Weatherhead, M. A. and M. R. James. 2001. Distribution of macroinvertebrates in relation to physical and biological variables in the littoral zone of nine New Zealand lakes. Hydrobiologia 462(1–3):115–129.
- ↑ Death, R. G., B. Baillie and P. Fransen. 2003. Effect of Pinus radiata logging on stream invertebrate communities in Hawke’s Bay, New Zealand. New Zealand Journal of Marine and Freshwater Research 37(3):507–520.
- ↑ Schreiber, E. S. G., G. P. Quinn and P. S. Lake. 2003. Distribution of an alien aquatic snail in relation to flow variability, human activities and water quality. Freshwater Biology 48(6):951–961.
- ↑ Suren, A. M. 2005. Effects of deposited sediment on patch selection by two grazing stream invertebrates. Hydrobiologia 549(1):205–218.
- ↑ Jacobsen, R. and V. E. Forbes. 1997. Clonal variation in life–history traits and feeding rates in the gastropod, Potamopyrgus antipodarum: performance across a salinity gradient. Functional Ecology 11(2):260–267.
- ↑ Leppäkoski, E. and S. Olenin. 2000. Non–native species and rates of spread: lessons from the brackish Baltic Sea. Biological Invasions 2(2):151–163.
- ↑ Costil, K., G.B. J. Dussart and J. Daquzan. 2001. Biodiversity of aquatic gastropods in the Mont St–Michel basin (France) in relation to salinity and drying of habitats. Biodiversity and Conservation 10(1):1–18.
- 1 2 Gerard, C., A. Blanc and K. Costil. 2003. Potamopyrgus antipodarum (Mollusca: Hydrobiidae) in continental aquatic gastropod communities: impact of salinity and trematode parasitism. Hydrobiologia 493(1–3):167–172.
- ↑ Cox, T. J. and J. C. Rutherford. 2000. Thermal tolerances of two stream invertebrates exposed to diurnally varying temperature. New Zealand Journal of Marine and Freshwater Research 34(2):203–208.
- ↑ Broekhuizen, N., S. Parkyn and D. Miller. 2001. Fine sediment effects on feeding and growth in the invertebrate grazer Potamopyrgus antipodarum (Gastropoda, Hydrobiidae) and Deleatidium sp. (Ephemeroptera, Letpophlebiidae). Hydrobiologia 457(1–3):125–132.
- ↑ James, M. R., I. Hawes and M. Weatherhead. 2000. Removal of settled sediments and periphyton from macrophytes by grazing invertebrates in the littoral zone of a large oligotrophic lake. Freshwater Biology 44(2):311–326.
- ↑ Kelly, D. J. and I. Hawes. 2005. Effects of invasive macrophytes on littoral–zone productivity and foodweb dynamics in a New Zealand high–country lake. Journal of the North American Benthological Society 24(2):300–320.
- ↑ Parkyn, S. M., J. M. Quinn, T. J. Cox and N. Broekhuizen. 2005. Pathways of N and C uptake and transfer in stream food webs: an isotope enrichment experiment. Journal of the North American Benthological Society 24(4):955–975.
- ↑ Fox J., Dybdahl M., Jokela J., Lively C. (1996). Genetic structure of coexisting sexual and clonal subpopulations in a freshwater snail (Potamopyrgus antipodarum). Evolution. 50 (4): 1541-1548
- ↑ Schreiber, E. S. G., A. Glaister, G. P. Quinn and P. S. Lake. 1998. Life history and population dynamics of the exotic snail Potamopyrgus antipodarum (Prosobranchia: Hydrobiidae) in Lake Purrumbete, Victoria, Australia. Marine and Freshwater Research 49(1):73–78.
- ↑ Lively, C. M. and J. Jokela. 2002. Temporal and spatial distribution of parasites and sex in a freshwater snail. Evolutionary Ecology Research 4(2):219–226.
- ↑ "New Zealand mudsnail (Potamopyrgus antipodarum)". Kansas Department of Wildlife and Parks. 2006. Retrieved 2006-05-04.
- ↑ Larval Trematoda: Winterbourne
- ↑ Dybdahl, M. F. and A. C. Krist. 2004. Genotypic vs. condition effects on parasite–driven rare advantage. Journal of Evolutionary Biology 17(5):967–973.
- ↑ About Microphallus
- ↑ Aamio, K. and E. Bornsdorff. 1997. Passing the gut of juvenile flounder Platichthys flesus (L.) – differential survival of zoobenthic prey species. Marine Biology 129: 11–14.
- ↑ Levri, E. P. 1998. Perceived predation risk, parasitism, and the foraging behavior of a freshwater snail (Potamopyrgus antipodarum). Canadian Journal of Zoology 76(10):1878–1884.
Further reading
- Kerans, B. L, M. F. Dybdahl, M. M. Gangloff and J. E. Jannot. 2005. Potamopyrgus antipodarum: distribution, density, and effects on native macroinvertebrate assemblages in the Greater Yellowstone ecosystem. Journal of the North American Benthological Society 24(1):123–138.
- Strzelec, M. 2005. Impact of the introduced Potamopyrgus antipodarum (Gastropods) on the snail fauna in post–industrial ponds in Poland. Biologia (Bratislava) 60(2):159–163.
External links
| Wikimedia Commons has media related to Potamopyrgus antipodarum. |
- C. Vareille-Morel, Resistance of the prosobranch mollusc Potamopyrgus jenkinsi (E.A. Smith, 1889) to decreasing temperatures : an experimental study; Annls Limnol. Volume 21, Number 3, 1985
- Potamopyrgus antipodarum at Animalbase taxonomy,short description, distribution, biology,status (threats), images
- Pesticides Database - Chemical Toxicity Studies - ,
- CISR - New Zealand Mud Snail Center for Invasive Species Research, Summary of New Zealand Mud Snail
- Species Profile- New Zealand Mud Snail (Potamopyrgus antipodarum), National Invasive Species Information Center, United States National Agricultural Library. Lists general information and resources for New Zealand Mud Snail.
