Pfitzner–Moffatt oxidation
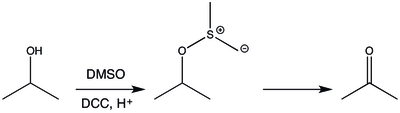
The Pfitzner–Moffatt oxidation, sometimes referred to as simply the Moffatt oxidation, is a chemical reaction which describes the oxidation of primary and secondary alcohols by dimethyl sulfoxide (DMSO) activated with a carbodiimide, such as dicyclohexylcarbodiimide (DCC). The resulting alkoxysulfonium ylide rearranges to generate aldehydes and ketones, respectively.[1][2]
This reaction has been largely abandoned for the Swern oxidation, which gives higher yields with fewer side products. The Moffatt oxidation yields urea by-products that are often difficult to remove.
Several reviews have been published.[3][4]
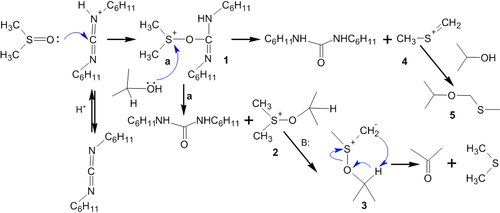
Reaction mechanism is as follows.
See also
References
- ↑ Pfitzner, K. E.; Moffatt, J. G. (1963). "A New and Selective Oxidation of Alcohols". J. Am. Chem. Soc. 85: 3027. doi:10.1021/ja00902a036.
- ↑ J. G. Moffatt, “Sulfoxide-Carbodiimide and Related Oxidations” in Oxidation vol. 2, R. L. Augustine, D. J. Trecker, Eds. (Dekker, New York, 1971) pp 1–64.
- ↑ Tidwell, T. T. Org. React. 1990, 39, 297–572. (Review)
- ↑ Lee, T. V. Comp. Org. Syn. 1991, 7, 291–303. (Review)
This article is issued from Wikipedia - version of the 9/29/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.