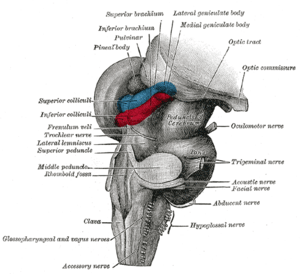
PGO waves
Ponto-geniculo-occipital waves or PGO waves are phasic field potentials. These waves can be recorded from the pons, the lateral geniculate nucleus (LGN), and the occipital cortex regions of the brain, where these waveforms originate. The waves begin as electrical pulses from the pons, then move to the lateral geniculate nucleus residing in the thalamus, and then finally end up in the primary visual cortex of the occipital lobe. The appearances of these waves are most prominent in the period right before rapid eye movement sleep (or REM sleep), and are theorized to be intricately involved with eye movement of both wake and sleep cycles in many different animals.
Discovery
The discovery of PGO waves goes back to 1959, when three French scientists released their scientific article of their study of these waves in animal test subjects.[1] Although at this time, they did not have a specific name for this neurological phenomenon.
It was not until the published work of two American scientists that these waves became known as PGO waves.[2] Their research focused on the propagation of these waves in cats, noticing that these field potentials started in the pons, propagating down to the lateral geniculate nucleus and the occipital lobe.
Other studies with these waves have been done on rats as well. Scientists tried to discern whether the rats had PGO waves, but learned that they are present only in the pons, and wave propagation does not excite any neurons in the lateral geniculate nucleus.[3] As a result of this study, PGO waves are known as P waves in rodents.
PGO waves have been studied mostly through cat and rodent animal models. Despite the focus of the research, PGO waves have been found to exist in other mammalian species including humans and nonhuman primates, such as the macaque and baboon.[4]
Detection
In the original experiments, PGO waves (or P waves in rodent models) are found by placing electrodes inside the brain, next to either the pons, lateral geniculate nuclei, or occipital lobe. Along with electroencephalography (EEG) recording techniques, scientists are also able to show the correlation between other brain waves associated with REM sleep and PGO waves.
Although scientists know they exist, PGO waves have not been detected in healthy humans due to the ethical concerns about accessing these areas where the readings need to be taken from. However, advances in deep brain stimulation has made it possible to put electrodes inside the brains of humans with different pathologies and make EEG recordings of different nuclei. Due to the similarities with the animal models, we can infer that PGO waves are happening at the same frequency in human EEGs.[5][6] Thus, scientists can infer that PGO waves exist in humans.
Mechanism for generation and propagation
The neurophysiological studies on PGO waves conclude that the generation of these waves reside in a collection of neurons located in the pons, regardless of species research is done on.[7] From this point, the neurons branch out in a network that leads the phasic electrical signal toward the lateral geniculate nucleus and the occipital lobe.
Within this network, there are two types of neuronal groups: executive neurons and modulatory neurons.
Executive neurons
These neurons are the ones that help to generate and propagate the PGO waves throughout the brain. One research paper further breaks down this "class" of neurons into two subsets: triggering neurons and transfer neurons.[4] All of these neurons are located in the peribrachial area, which is a group of neurons surrounding the superior cerebellar penduncle.
Triggering neurons
These neurons are located in the caudolateral region of the peribrachial area. These neurons actively fire during non-REM (NREM) sleep. The most recorded activity of the neurons is during the N3 stage of NREM, also known as the slow-wave sleep cycle. These same neurons are also active during REM sleep, but at a greatly reduced amplitude than NREM sleep.[7]
Transfer neurons
The neuronal cells that allow for the transfer of PGO waves from the pons to the other parts of the brain reside on the rostral portion of the peribrachial area. This grouping of cells fire in precisely two modes. The first mode is burst firing through low-threshold Calcium (Ca2+) ion channels. The other mode is a repetitive tonic firing through Sodium (Na+) dependent ion channels.[8]
During the times when triggering neurons are firing, these cells receive those signals and begin increasing their firing. This, in turn, allows the wave to go out to the other portions of the brain.
Modulatory neurons
As the executive neurons are firing, the spread of the wave is controlled by both excitatory and inhibitory inputs. These inputs come from the modulatory neurons, which help to regulate and control the amplitude and frequency of the wave. The following types of cells play a huge part in this control process.
Aminergic neurons
Aminergic neurons are neurons that use monoamines as a neurotransmitter. This class of neurotransmitters is what keeps PGO wave amplitudes at very low levels during periods of a mammal being awake. The two specific aminergic neurotransmitters are serotonin and norepinephrine.[9]
Cholinergic neurons
Cholinergic neurons are neurons that use acetylcholine as a neurotransmitter. Through different studies, these types of neurons have been proven to promote PGO wave generation, thus being an excitatory neuromodulator for triggering neurons.[10]
Nitroxergic neurons
Nitroxergic neurons use nitric oxide (NO) as a neurotransmitter. In theory, the increase of nitric oxide is seen as an excitatory neuromodulator in PGO wave generation.[4] This stems from animal testing that has shown increases in PGO waves as nitric oxide levels were increased in the pons.[11]
GABA-ergic neurons
GABA-ergic neurons use gamma-aminobutyric acid (GABA) as a neurotransmitter. These neurons are theorized to be inhibitory to aminergic neurons, and thus inhibitory to PGO wave propagation.[4]
Vestibular nuclei
The neurons within the vestibular nuclei region of the brain have been shown to provide excitatory bouts of PGO wave generation when stimulated.[12] The tests showed that, while the vestibular nuclei aided in creating PGO waves, the excitation of this area of the brain was in no way needed for PGO wave formation.
Amygdala
The neurons within the amygdala region of the brain have also been shown to provide excitatory bouts of PGO wave generation when electrically stimulated.[13]
Suprachiasmatic nuclei
The neurons within the suprachiasmatic nuclei region of the brain help to regulate REM sleep.[14] The REM sleep cycle length causes the frequency of PGO waves to be phase locked.
Auditory stimulation
The use of auditory stimulation has been shown to increase PGO waves during waking and sleeping cycles with neurons associated with transfers of auditory information.[15] Even while the subject is awake and in total darkness, the amplitude of PGO waves increases by auditory stimulation. Another study also found that auditory stimulation increased the amplitude of PGO waves in slow-wave sleep and REM sleep and did not reduce the amplitude of the waves with repeated auditory stimulation.[16] From this research, scientists can theorize that PGO wave generation from auditory stimulation contains a positive-feedback mechanism that can be excited by evoked PGO waves.[4]
Basal ganglia
The basal ganglia are a group of nuclei in the brains of vertebrates, situated at the base of the forebrain and strongly connected with the cerebral cortex, thalamus and pons. The basal ganglia are associated with a variety of functions, including arousal, motor control and learning. The main components of the basal ganglia are the striatum, pallidum, substantia nigra, and subthalamic nucleus (or subthalamus). This latter, glutamatergic nucleus is reciprocally connected with the PGO-transferring nuclei of the pons. In humans, subthalamic PGO-like waves, that resemble the PGO waves typically recorded in cats, can be recorded during pre-REM and REM sleep.[17] This suggests that the subthalamus may play an active role in an ascending activating network implicated in the rostral transmission of PGO waves during REM sleep in humans.[17]
REM sleep
PGO waves are an integral part of rapid eye movement (REM) sleep. As stated earlier, the density of the PGO waves coincides with the amount of eye movement measured in REM sleep. This has led some researchers to further theorize about the usefulness of PGO waves for dreaming.
One key use of REM sleep is for the brain to process and store information from the previous day. In a sense, the brain is learning by establishing new neuronal connections for things that have been learned. Neurophysiological studies have indicated a relationship between increased P-wave density during post-training REM sleep and learning performance.[18][19] Basically, the abundance of PGO waves translates into longer periods of REM sleep, which thereby allows the brain to have longer periods where neuronal connections are formed.
The importance of PGO waves during REM sleep also aids the idea of PGO waves as a signal that a person is dreaming.[20] Since dreaming occurs during REM sleep, the PGO waves are theorized to be the signals that make the brain start to recount the experiences from the previous day. This, in turn, allows us to "see" our dreams since our visual sense is quickly going through the information it has stored.
For more information of the importance of PGO waves during REM sleep, please refer to Activation synthesis theory.
Future research
The next big area to research regarding this topic is to understand what processes PGO waves are beneficial for in both states of sleep as well as consciousness. Although PGO waves are noticeably present during NREM sleep, some scientists believe they are also present during waking cycles. There is a possibility that PGO waves are essential for image correction of the eyes, since PGO waves are signals on a pathway that allow the motor cortex of the brain to interface with the vision system.
The other future interests in research of this topic are what PGO waves are precisely doing for us while we are dreaming. Some scientists theorize that PGO waves are essential for stabilizing the images that we have in our dreams. This theory comes from the eye movements that coincide with the readings from electrodes picking up PGO waves.
Also, scientists seek to discover how the presence of these signals fits in with the overall dreaming process. Research in PGO waves is of broad importance to neurophysiology because it involves the coordination of entire regions of the brain working together—coming from many different systems—to create our dreams, memories, and other experiences that we have.
Another area of potential research interest involves PGO waves during lucid dreaming.
Additional images
| Human brain photos | ||||||
|---|---|---|---|---|---|---|
|
See also
References
- ↑ Jouvet, M., Michel, F., and Courjon, J. 1959. L'activite electrique du rhinencephale au cours dusommeil chez le chat. C.R. Soc. Biol. 153:101–105.
- ↑ Brooks, D.C., and Bizzi, E. 1963. Brain stem electrical activity during deep sleep. Arch. Ital. Biol.101:648–665.
- ↑ Stern, W.C., Forbes, W.B., and Morgane, P.J. 1974. Absence of ponto-geniculo-occipital (PGO) spikes in rats. Physiol. Behav. 12:293–295.
- 1 2 3 4 5 Datta S. 1997. Cellular basis of pontine ponto-geniculo-occipital wave generation and modulation. Cellular and Molecular Neurobiology 17:341–65
- ↑ Fernández-Mendoza J., Lozano B., Seijo F., Fernández-González F., Vela-Bueno A. 2006. Subthalamic nucleus activity during human REM sleep: the PGO-like waves. J Sleep Res 2006;15:243.
- ↑ Lim A.S., Lozano A.M., Moro E., Hamani C., Hutchison W.D., et al. 2007b. Characterization of REM-sleep associated ponto-geniculo-occipital waves in the human pons. Sleep 30:823–7
- 1 2 Datta, S., and Hobson, J.A. 1994. Neuronal activity in the caudo-lateral peribrachial pons:Relationship to PGO waves and rapid eye movements. J. Neurophysiol. 71:95–109.
- ↑ Williams, J.A., and Reiner, P.B. 1993. Noradrenaline hyperpolarizes identified rat mesopontine cholinergic neurons in vitro. J. Neurosci. 13:3878–3883.
- ↑ Brooks, D. C.; Gershon, M. D. (1977-01-01). "Amine repletion in the reserpinized cat: effect upon PGO waves and REM sleep". Electroencephalography and Clinical Neurophysiology. 42 (1): 35–47. doi:10.1016/0013-4694(77)90149-3. ISSN 0013-4694. PMID 64348.
- ↑ Steriade, M., Datta, S., Pare, D., Oakson, G., and Currodossi, R. (1990a). Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamortical systems. J. Heurosci. 10:2541–2559.
- ↑ Leonard, T.O., and Lydic, R. 1995. Nitric oxide synthase inhibition decreases pontine acetylcholinerelease. NeuroReport 6:1525–1529.
- ↑ Morrison, A.R., and Pompeiano, O. (1966). Vestibular influences during sleep. IV. Functional relations between the vestibular nsleep. Arch. Ital. Biol. 104:425–458.
- ↑ Calvo, J.M., Badillo, S., Morales-Ramirez, M., and Palacios-Salas, P. (1987). The role of temporal lobe amygdala in ponto-geniculo-occipital activity and sleep organization in cats. Brain Res. 403:22–30.
- ↑ Siegel J.M. 2005. Clues to the functions of mammalian sleep. Nature 437:1264–71
- ↑ Callaway C.W., Lydic R., Baghdoyan H.A., Hobson J.A. 1987. Pontogeniculoocipital Waves – Spontaneous Visual-System ACtivity During Rapid Eye-Movement Sleep. Cellular and Molecular Neurobiology 7:105–49
- ↑ Bowker, R.M., and Morrison, A.R. 1977. The PGO spikes: An indicator of hyperalertness. In Sleep Research, (W.P. Koella and P. Levin, Eds.), Karger, Basel, pp. 23–77.
- 1 2 Fernández-Mendoza J., Lozano B., Seijo F., Santamarta-Liébana E., Ramos-Platón M.J., Vela-Bueno A., Fernández-González F. 2009. Evidence of subthalamic pgo-like waves during rem sleep in humans: a deep brain polysomnographic study. SLEEP 32(9):1117–26.
- ↑ Datta, S., 2006. Activation of phasic pontine-wave generator: a mechanism for sleep-dependent memory processing. Sleep Biol. Rhythms 4, 16–26.
- ↑ Datta, S., Saha, S., Prutzman, S.L., Mullins, O.J., Mavanji, V., 2005. Pontine-wave generator activation-dependent memory processing of avoidance learning involves the dorsal hippocampus in the rat. J. Neurosci. Res. 80, 727–737. Sleep Biol. Rhythms 4, 44–54.
- ↑ Hobson J.A., Pace-Schott E.F., Stickgold R. 2000. Dreaming and the brain: Toward a cognitive neuroscience of conscious states. Behavioral and Brain Sciences 23:793–+