Oral cancer
| Oral cancer | |
|---|---|
|
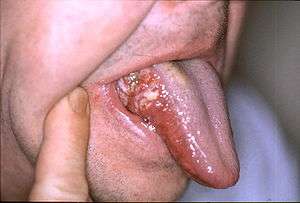
Oral cancer on the side of the tongue, a common site along with the floor of the mouth | |
| Classification and external resources | |
| Specialty | Oncology, otorhinolaryngology |
| ICD-10 | C00-C08 |
| ICD-9-CM | 140-146 |
| DiseasesDB | 9288 |
| MedlinePlus | 001035 |
| MeSH | D009062 |
Oral cancer, also known as mouth cancer,[1] is a type of head and neck cancer and is any cancerous tissue growth located in the oral cavity.[2]
It may arise as a primary lesion originating in any of the tissues in the mouth, by metastasis from a distant site of origin, or by extension from a neighboring anatomic structure, such as the nasal cavity. Alternatively, the oral cancers may originate in any of the tissues of the mouth, and may be of varied histologic types: teratoma, adenocarcinoma derived from a major or minor salivary gland, lymphoma from tonsillar or other lymphoid tissue, or melanoma from the pigment-producing cells of the oral mucosa. There are several types of oral cancers, but around 90% are squamous cell carcinomas,[3] originating in the tissues that line the mouth and lips. Oral or mouth cancer most commonly involves the tongue. It may also occur on the floor of the mouth, cheek lining, gingiva (gums), lips, or palate (roof of the mouth). Most oral cancers look very similar under the microscope and are called squamous cell carcinoma, but less commonly other types of oral cancer occur, such as Kaposi's sarcoma.
In 2013 oral cancer resulted in 135,000 deaths up from 84,000 deaths in 1990.[4] Five-year survival rates in the United States are 63%.[5]
Signs and symptoms
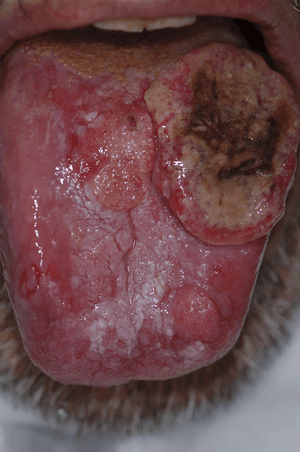
In its early stages, it can go unnoticed. It can be painless with slight physical changes. But the precursor tissue changes, can be noticed by the doctors.
Early stage symptoms can include persistent red or white patches, a non-healing ulcer, progressive swelling or enlargement, unusual surface changes, sudden tooth mobility without apparent cause, unusual oral bleeding or epitaxis and prolonged hoarseness.[6]
Late stage symptoms can include an indurated area, paresthesia or dysesthesia of the tongue or lips, airway obstruction, chronic serous otitis media, otalgia, trismus, dysphagia, cervical lymphadenopathy, persistent pain or referred pain and altered vision.[6]
Causes
Oncogenes are activated as a result of mutation of the DNA. Risk factors that predispose a person to oral cancer have been identified in epidemiological (epidemiology) studies.
Around 75 percent of oral cancers are linked to modifiable behaviors such as tobacco use and excessive alcohol consumption. Other factors include poor oral hygiene, irritation caused by ill-fitting dentures and other rough surfaces on the teeth, poor nutrition, and some chronic infections caused by fungi, bacteria or viruses.[7] If oral cancer is diagnosed in its earliest stages, treatment is generally very effective.
Chewing betel, paan and Areca is known to be a strong risk factor for developing oral cancer. In India where such practices are common, oral cancer represents up to 40% of all cancers, compared to just 4% in the UK.
Oral cancer often presents as a non-healing ulcer (shows no sign of healing after 2 weeks). In the US oral cancer accounts for about 8 percent of all malignant growths. Men are affected twice as often as women, particularly men older than 40/60.
Premalignant lesions
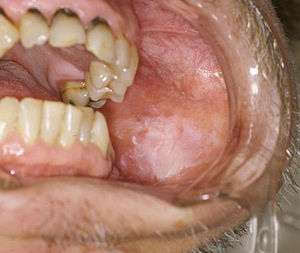
A premalignant (or precancerous) lesion is defined as "a benign, morphologically altered tissue that has a greater than normal risk of malignant transformation." There are several different types of premalignant lesion that occur in the mouth. Some oral cancers begin as white patches (leukoplakia), red patches (erythroplakia) or mixed red and white patches (erythroleukoplakia or "speckled leukoplakia"). Other common premalignant lesions include oral lichen planus (particularly the erosive type), oral submucous fibrosis and actinic cheilitis.[9] In the Indian subcontinent oral submucous fibrosis is very common. This condition is characterized by limited opening of mouth and burning sensation on eating of spicy food. This is a progressive lesion in which the opening of the mouth becomes progressively limited, and later on even normal eating becomes difficult. It occurs almost exclusively in India and Indian communities living abroad. The overall prevalence of oral potentially malignant disorders in the Middle East was 2.8%. Lichen planus/lichenoid lesions were the most common lesions (1.8%) followed by leukoplakias (0.48%), chronic hyperplastic candidiosis (0.38%), and erythroplakia (0.096%). Smoking, alcohol, and age (>40 years) were the main identifiable risk factors.[10]
Tobacco

In a study of Europeans, smoking and other tobacco use was associated with about 75 percent of oral cancer cases,[11] caused by irritation of the mucous membranes of the mouth from smoke and heat of cigarettes, cigars, and pipes. Tobacco contains over 60 known carcinogens, and the combustion of it, and by-products from this process, is the primary mode of involvement. Use of chewing tobacco or snuff causes irritation from direct contact with the mucous membranes.
Tobacco use in any form by itself, and even more so in combination with heavy alcohol consumption, continues to be an important risk factor for oral cancer. However, due to the current trends in the spread of HPV16, as of early 2011 the virus is now considered the primary causative factor in 63% of newly diagnosed patients.
Alcohol
Some studies in Australia, Brazil and Germany pointed to alcohol-containing mouthwashes as also being etiologic agents in the oral cancer risk family. The claim was that constant exposure to these alcohol-containing rinses, even in the absence of smoking and drinking, leads to significant increases in the development of oral cancer. However, studies conducted in 1985,[12] 1995,[13] and 2003[14] summarize that alcohol-containing mouth rinses are not associated with oral cancer. In a March 2009 brief, the American Dental Association said "the available evidence does not support a connection between oral cancer and alcohol-containing mouthrinse".[15] A 2008 study suggests that acetaldehyde (a breakdown product of alcohol) is implicated in oral cancer,[16][17] but this study specifically focused on abusers of alcohol and made no reference to mouthwash. Any connection between oral cancer and mouthwash is tenuous without further investigation.
Human papillomavirus
Infection with human papillomavirus (HPV), particularly type 16 (there are over 180 types), is a known risk factor and independent causative factor for oral cancer.[18] A fast-growing segment of those diagnosed does not present with the historic stereotypical demographics. Historically that has been people over 50, blacks over whites 2 to 1, males over females 3 to 1, and 75% of the time people who have used tobacco products or are heavy users of alcohol. This new and rapidly growing sub population between 30 and 50 years old,[19] is predominantly nonsmoking, white, and males slightly outnumber females. Recent research from multiple peer-reviewed journal articles indicates that HPV16 is the primary risk factor in this new population of oral cancer victims. HPV16 (along with HPV18) is the same virus responsible for the vast majority of all cervical cancers and is the most common sexually transmitted infection in the US. Oral cancer in this group tends to favor the tonsil and tonsillar pillars, base of the tongue, and the oropharynx. Recent data suggest that individuals that come to the disease from this particular etiology have a significant survival advantage,[20] as the disease responds better to radiation treatments than tobacco etiology disease.
Hematopoietic stem cell transplantation
Patients after hematopoietic stem cell transplantation (HSCT) are at a higher risk for oral squamous cell carcinoma. Post-HSCT oral cancer may have more aggressive behavior with poorer prognosis, when compared to oral cancer in non-HSCT patients.[21] This effect is supposed to be owing to the continuous lifelong immune suppression and chronic oral graft-versus-host disease.[21]
Diagnosis

_squamous_cell_carcinoma_histopathology.jpg)
Early diagnosis of oral cancer patients would decrease mortality and help to improve treatment. Oral surgeons and dentists are the early diagnosers that diagnose these patients in early stages. Health providers, dentists, and oral surgeons shall have high knowledge and awareness that would help them to provide better diagnosis for oral cancer patients. An examination of the mouth by the health care provider, dentist, oral surgeons shows a visible and/or palpable (can be felt) lesion of the lip, tongue, or other mouth area. The lateral/ventral sides of the tongue are the most common sites for intraoral SCC. As the tumor enlarges, it may become an ulcer and bleed. Speech/talking difficulties, chewing problems, or swallowing difficulties may develop. A feeding tube is often necessary to maintain adequate nutrition. This can sometimes become permanent as eating difficulties can include the inability to swallow even a sip of water. The doctor can order some special investigations which may include a chest x-ray, CT or MRI scans, and tissue biopsy.
There are a variety of screening devices that may assist dentists in detecting oral cancer, including the Velscope, Vizilite Plus and the identafi 3000. There is no evidence that routine use of these devices in general dental practice saves lives.[22] However, there are compelling reasons to be concerned about the risk of harm this device may cause if routinely used in general practice. Such harms include false positives, unnecessary surgical biopsies and a financial burden on the patient. While a dentist, physician or other health professional may suspect a particular lesion is malignant, there is no way to tell by looking alone - since benign and malignant lesions may look identical to the eye. A non-invasive brush biopsy (BrushTest) can be performed to rule out the presence of dysplasia (pre-cancer) and cancer on areas of the mouth that exhibit an unexplained color variation or lesion. The only definitive method for determining if cancerous or precancerous cells are present is through biopsy and microscopic evaluation of the cells in the removed sample. A tissue biopsy, whether of the tongue or other oral tissues and microscopic examination of the lesion confirm the diagnosis of oral cancer or precancer. There are six common species of bacteria found at significantly higher levels in the saliva of patients with oral squamous cell carcinoma (OSCC) than in saliva of oral-free cancer individuals. Three of the six, C. gingivalis, P. melaninogenica, and S. mitis, can be used as a diagnostic tool to predict more than 80% of oral cancers.[23]
Management
Surgical excision (removal) of the tumor is usually recommended if the tumor is small enough, and if surgery is likely to result in a functionally satisfactory result. Radiation therapy with or without chemotherapy is often used in conjunction with surgery, or as the definitive radical treatment, especially if the tumour is inoperable. Surgeries for oral cancers include:
- Maxillectomy (can be done with or without orbital exenteration)
- Mandibulectomy (removal of the mandible or lower jaw or part of it)
- Glossectomy (tongue removal, can be total, hemi or partial). When glossectomy is performed for smaller tumors (< 4 cm), the adequacy of resection (margin status) is best assessed from the resected specimen itself. The status of the margin (positive/tumor cut through versus negative/clear margin) obtained from the glossectomy specimen appears to be of prognostic value, while the status of the margin sampled from the post-glossectomy defect is not. The method of margin sampling appears to correlate with local recurrence: preference for tumor bed/defect margins may be associated with worse local control.[24][25]
- Radical neck dissection
- Mohs surgery or CCPDMA
- Combinational, e.g. glossectomy and laryngectomy done together
- Feeding tube to sustain nutrition
Owing to the vital nature of the structures in the head and neck area, surgery for larger cancers is technically demanding. Reconstructive surgery may be required to give an acceptable cosmetic and functional result. Bone grafts and surgical flaps such as the radial forearm flap are used to help rebuild the structures removed during excision of the cancer. An oral prosthesis may also be required. Most oral cancer patients depend on a feeding tube for their hydration and nutrition. Some will also get a port for the chemo to be delivered. Many oral cancer patients are disfigured and suffer from many long term after effects. The after effects often include fatigue, speech problems, trouble maintaining weight, thyroid issues, swallowing difficulties, inability to swallow, memory loss, weakness, dizziness, high frequency hearing loss and sinus damage.
Survival rates for oral cancer depend on the precise site, and the stage of the cancer at diagnosis. Overall, 2011 data from the SEER database shows that survival is around 57% at five years when all stages of initial diagnosis, all genders, all ethnicities, all age groups, and all treatment modalities are considered. Survival rates for stage 1 cancers are approximately 90%, hence the emphasis on early detection to increase survival outcome for patients. Similar survival rates are reported from other countries such as Germany.[26]
Following treatment, rehabilitation may be necessary to improve movement, chewing, swallowing, and speech. Speech and language pathologists may be involved at this stage.
Chemotherapy is useful in oral cancers when used in combination with other treatment modalities such as radiation therapy. It is not used alone as a monotherapy. When cure is unlikely it can also be used to extend life and can be considered palliative but not curative care. Biological agents, such as Cetuximab have recently been shown to be effective in the treatment of squamous cell head and neck cancers, and are likely to have an increasing role in the future management of this condition when used in conjunction with other established treatment modalities.
Treatment of oral cancer will usually be by a multidisciplinary team, with treatment professionals from the realms of radiation, surgery, chemotherapy, nutrition, dental professionals, and even psychology all possibly involved with diagnosis, treatment, rehabilitation, and patient care.
Prognosis
- Postoperative disfigurement of the face, head and neck
- Complications of radiation therapy, including dry mouth and difficulty swallowing
- Other metastasis (spread) of the cancer
- Significant weight loss
Prognosis depends on stage and overall health. Grading of the invasive front of the tumor is a very important prognostic parameter.[27]
Epidemiology
In 2013 oral cancer resulted in 135,000 deaths up from 84,000 deaths in 1990.[4] Oral cancer occurs more often in people from the lower end of the socioeconomic scale.[28]
In 2011, close to 37,000 Americans are projected to be diagnosed with oral or pharyngeal cancer. 66% of the time these will be found as late stage three and four disease. It will cause over 8,000 deaths. Of those 37,000 newly diagnosed individuals, only slightly more than half will be alive in 5 years. Similar survival estimates are reported from other countries. For example, five-year relative survival for oral cavity cancer patients in Germany is about 55%.[26] Survival rates of patients diagnosed with oral cancer have not significantly improved in decades. The death rate for oral cancer is higher than cervical cancer, Hodgkin's lymphoma, laryngeal cancer, cancer of the testes, and endocrine system cancers such as thyroid, or skin cancer (malignant melanoma). If the definition of oral cancer is expanded to include cancer of the larynx, for which the risk factors are the same, the numbers of diagnosed cases grow to approximately 50,000 individuals, and 13,500 deaths per year in the U.S.. Worldwide, the problem is much greater, with over 640,000 new cases being found each year.[29]
Low public awareness of the disease is a significant factor, but these cancers could be found at early highly survivable stages through a simple, painless, five-minute examination by a trained medical or dental professional.
UK
Oral cancer is the sixteenth most common cancer in the UK (around 6,800 people were diagnosed with oral cancer in the UK in 2011), and it is the nineteenth most common cause of cancer death (around 2,100 people died from the disease in 2012).[30]
India
Oral cancer is the most common form of cancer in India. 130,000 people succumb to oral cancer in India annually. The reason for this high prevalence of oral cancer in India is primarily tobacco consumed in the form of gutka, quid, snuff or misri.[31] In the North East India, the use of areca nut is also a risk factor for oral cancer.
See also
References
- ↑ Lozano, Rafael; Naghavi, Mohsen; Foreman, Kyle; Lim, Stephen; Shibuya, Kenji; Aboyans, Victor; Abraham, Jerry; Adair, Timothy; Aggarwal, Rakesh; Ahn, Stephanie Y; AlMazroa, Mohammad A; Alvarado, Miriam; Anderson, H Ross; Anderson, Laurie M; Andrews, Kathryn G; Atkinson, Charles; Baddour, Larry M; Barker-Collo, Suzanne; Bartels, David H; Bell, Michelle L; Benjamin, Emelia J; Bennett, Derrick; Bhalla, Kavi; Bikbov, Boris; Abdulhak, Aref Bin; Birbeck, Gretchen; Blyth, Fiona; Bolliger, Ian; Boufous, Soufiane; Bucello, Chiara; Burch, Michael; Burney, Peter; Carapetis, Jonathan; Chen, Honglei; Chou, David; Chugh, Sumeet S; Coffeng, Luc E; Colan, Steven D; Colquhoun, Samantha; Colson, K Ellicott; Condon, John; Connor, Myles D; Cooper, Leslie T; Corriere, Matthew; Cortinovis, Monica; de Vaccaro, Karen Courville; Couser, William; Cowie, Benjamin C; Criqui, Michael H; Cross, Marita; Dabhadkar, Kaustubh C; Dahodwala, Nabila; De Leo, Diego; Degenhardt, Louisa; Delossantos, Allyne; Denenberg, Julie; Des Jarlais, Don C; Dharmaratne, Samath D; Dorsey, E Ray; Driscoll, Tim; Duber, Herbert; Ebel, Beth; Erwin, Patricia J; Espindola, Patricia; Ezzati, Majid; Feigin, Valery; Flaxman, Abraham D; Forouzanfar, Mohammad H; Fowkes, Francis Gerry R; Franklin, Richard; Fransen, Marlene; Freeman, Michael K; Gabriel, Sherine E; Gakidou, Emmanuela; Gaspari, Flavio; Gillum, Richard F; Gonzalez-Medina, Diego; Halasa, Yara A; Haring, Diana; Harrison, James E; Havmoeller, Rasmus; Hay, Roderick J; Hoen, Bruno; Hotez, Peter J; Hoy, Damian; Jacobsen, Kathryn H; James, Spencer L; Jasrasaria, Rashmi; Jayaraman, Sudha; Johns, Nicole; Karthikeyan, Ganesan; Kassebaum, Nicholas; Keren, Andre; Khoo, Jon-Paul; Knowlton, Lisa Marie; Kobusingye, Olive; Koranteng, Adofo; Krishnamurthi, Rita; Lipnick, Michael; Lipshultz, Steven E; Ohno, Summer Lockett; Mabweijano, Jacqueline; MacIntyre, Michael F; Mallinger, Leslie; March, Lyn; Marks, Guy B; Marks, Robin; Matsumori, Akira; Matzopoulos, Richard; Mayosi, Bongani M; McAnulty, John H; McDermott, Mary M; McGrath, John; Memish, Ziad A; Mensah, George A; Merriman, Tony R; Michaud, Catherine; Miller, Matthew; Miller, Ted R; Mock, Charles; Mocumbi, Ana Olga; Mokdad, Ali A; Moran, Andrew; Mulholland, Kim; Nair, M Nathan; Naldi, Luigi; Narayan, K M Venkat; Nasseri, Kiumarss; Norman, Paul; O'Donnell, Martin; Omer, Saad B; Ortblad, Katrina; Osborne, Richard; Ozgediz, Doruk; Pahari, Bishnu; Pandian, Jeyaraj Durai; Rivero, Andrea Panozo; Padilla, Rogelio Perez; Perez-Ruiz, Fernando; Perico, Norberto; Phillips, David; Pierce, Kelsey; Pope, C Arden; Porrini, Esteban; Pourmalek, Farshad; Raju, Murugesan; Ranganathan, Dharani; Rehm, Jürgen T; Rein, David B; Remuzzi, Guiseppe; Rivara, Frederick P; Roberts, Thomas; De León, Felipe Rodriguez; Rosenfeld, Lisa C; Rushton, Lesley; Sacco, Ralph L; Salomon, Joshua A; Sampson, Uchechukwu; Sanman, Ella; Schwebel, David C; Segui-Gomez, Maria; Shepard, Donald S; Singh, David; Singleton, Jessica; Sliwa, Karen; Smith, Emma; Steer, Andrew; Taylor, Jennifer A; Thomas, Bernadette; Tleyjeh, Imad M; Towbin, Jeffrey A; Truelsen, Thomas; Undurraga, Eduardo A; Venketasubramanian, N; Vijayakumar, Lakshmi; Vos, Theo; Wagner, Gregory R; Wang, Mengru; Wang, Wenzhi; Watt, Kerrianne; Weinstock, Martin A; Weintraub, Robert; Wilkinson, James D; Woolf, Anthony D; Wulf, Sarah; Yeh, Pon-Hsiu; Yip, Paul; Zabetian, Azadeh; Zheng, Zhi-Jie; Lopez, Alan D; Murray, Christopher JL (2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". The Lancet. 380 (9859): 2095–128. doi:10.1016/S0140-6736(12)61728-0. PMID 23245604.
- ↑ Werning, John W (May 16, 2007). Oral cancer: diagnosis, management, and rehabilitation. p. 1. ISBN 978-1-58890-309-9.
- ↑ http://www.oralcancerfoundation.org/facts/index.htm
- 1 2 GBD 2013 Mortality Causes of Death Collaborators (2015). "Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013". The Lancet. 385 (9963): 117–71. doi:10.1016/S0140-6736(14)61682-2. PMC 4340604
 . PMID 25530442.
. PMID 25530442. - ↑ "SEER Stat Fact Sheets: Oral Cavity and Pharynx Cancer". NCI. Retrieved 18 June 2014.
- 1 2 Ravikiran Ongole, Praveen B N, ed. (2014). Textbook of Oral Medicine, Oral Diagnosis and Oral Radiology. Elsevier India. p. 387. ISBN 978-8131230916.
- ↑ Srinivasprasad, Vijayan; Dineshshankar, Janardhanam; Sathiyajeeva, J; Karthikeyan, M; Sunitha, J; Ragunathan, Ramachandran (2015). "Liaison between micro-organisms and oral cancer". Journal of Pharmacy and Bioallied Sciences. 7 (Suppl 2): S354–60. doi:10.4103/0975-7406.163451. PMC 4606619
 . PMID 26538877.
. PMID 26538877. - ↑ Lodi, Giovanni; Sardella, Andrea; Bez, Cristina; Demarosi, Federica; Carrassi, Antonio; Lodi, Giovanni (2006). "Interventions for treating oral leukoplakia". The Cochrane Database of Systematic Reviews (4): CD001829. doi:10.1002/14651858.CD001829.pub3. PMID 17054142.
- ↑ Neville BW, Damm DD, Allen CM, Bouquot JE (2002). Oral & maxillofacial pathology (2nd ed.). Philadelphia: W.B. Saunders. pp. 337, 345, 349, 353. ISBN 0721690033.
- ↑ Hassona, Y; Scully, C; Almangush, A; Baqain, Z; Sawair, F (2014). "Oral potentially malignant disorders among dental patients: a pilot study in Jordan". Asian Pacific Journal of Cancer Prevention : APJCP. 15 (23): 10427–31. PMID 25556487.
- ↑ Rodriguez, Teresa; Altieri, Andrea; Chatenoud, Liliane; Gallus, Silvano; Bosetti, Cristina; Negri, Eva; Franceschi, Silvia; Levi, Fabio; Talamini, Renato; La Vecchia, Carlo (2004). "Risk factors for oral and pharyngeal cancer in young adults". Oral Oncology. 40 (2): 207–13. doi:10.1016/j.oraloncology.2003.08.014. PMID 14693246.
- ↑ Mashberg, Arthur; Barsa, Patrice; Grossman, Martin L. (1985). "A study of the relationship between mouthwash use and oral and pharyngeal cancer". The Journal of the American Dental Association. 110 (5): 731–4. doi:10.14219/jada.archive.1985.0422. PMID 3859544.
- ↑ Elmore, J; Horwitz, R (1995). "Oral cancer and mouthwash use: Evaluation of the epidemiologic evidence". Otolaryngology - Head and Neck Surgery. 113 (3): 253–61. doi:10.1016/S0194-5998(95)70114-1. PMID 7675486.
- ↑ Cole, Philip; Rodu, Brad; Mathisen, Annette (2003). "Alcohol-containing mouthwash and oropharyngeal cancer". The Journal of the American Dental Association. 134 (8): 1079–87. doi:10.14219/jada.archive.2003.0322. PMID 12956348.
- ↑ Science brief on alcohol-containing mouthrinses and oral cancer, Archived March 19, 2012, at the Wayback Machine. American Dental Association, March 2009
- ↑ Warnakulasuriya, Saman; Parkkila, Seppo; Nagao, Toru; Preedy, Victor R.; Pasanen, Markku; Koivisto, Heidi; Niemelä, Onni (2007). "Demonstration of ethanol-induced protein adducts in oral leukoplakia (pre-cancer) and cancer". Journal of Oral Pathology & Medicine. 37 (3): 157–65. doi:10.1111/j.1600-0714.2007.00605.x.
- ↑ Alcohol and oral cancer research breakthrough Archived May 2, 2009, at the Wayback Machine.
- ↑ Gillison et al. Johns Hopkins
- ↑ Martin-Hernan, F.; Sanchez-Hernandez, JG.; Cano, J.; Campo, J.; del Romero, J. (2013). "Oral cancer, HPV infection and evidence of sexual transmission". Medicina Oral Patología Oral y Cirugia Bucal. 18 (3): e439–44. doi:10.4317/medoral.18419. PMC 3668870
 . PMID 23524417.
. PMID 23524417. - ↑ HPV-Positive Tumor Status Indicates Better Survival in Patients with Oropharyngeal Cancer
- 1 2 Elad, Sharon; Zadik, Yehuda; Zeevi, Itai; Miyazaki, Akihiro; De Figueiredo, Maria A. Z.; Or, Reuven (2010). "Oral Cancer in Patients After Hematopoietic Stem-Cell Transplantation: Long-Term Follow-Up Suggests an Increased Risk for Recurrence". Transplantation. 90 (11): 1243–4. doi:10.1097/TP.0b013e3181f9caaa. PMID 21119507.
- ↑ http://www.cochrane.org/CD004150/ORAL_screening-programmes-for-the-early-detection-and-prevention-of-oral-cancer/
- ↑ Mager, DL; Haffajee, AD; Devlin, PM; Norris, CM; Posner, MR; Goodson, JM (2005). "The salivary microbiota as a diagnostic indicator of oral cancer: a descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects". Journal of Translational Medicine. 3: 27. doi:10.1186/1479-5876-3-27. PMC 1226180
 . PMID 15987522.
. PMID 15987522. - ↑ Varvares, MA; Poti, S; Kenyon, B; Christopher, K; Walker, RJ (October 2015). "Surgical margins and primary site resection in achieving local control in oral cancer resections.". The Laryngoscope. 125 (10): 2298–307. doi:10.1002/lary.25397. PMID 26011037.
- ↑ Maxwell, JH; Thompson, LD; Brandwein-Gensler, MS; Weiss, BG; Canis, M; Purgina, B; Prabhu, AV; Lai, C; Shuai, Y; Carroll, WR; Morlandt, A; Duvvuri, U; Kim, S; Johnson, JT; Ferris, RL; Seethala, R; Chiosea, SI (1 December 2015). "Early Oral Tongue Squamous Cell Carcinoma: Sampling of Margins From Tumor Bed and Worse Local Control.". JAMA otolaryngology-- head & neck surgery. 141 (12): 1104–10. doi:10.1001/jamaoto.2015.1351. PMID 26225798.
- 1 2 Listl, S.; Jansen, L.; Stenzinger, A.; Freier, K.; Emrich, K.; Holleczek, B.; Katalinic, A.; Gondos, A.; Brenner, H.; GEKID Cancer Survival Working Group (2013). Scheurer, Michael, ed. "Survival of Patients with Oral Cavity Cancer in Germany". PLoS ONE. 8 (1): e53415. doi:10.1371/journal.pone.0053415. PMC 3548847
 . PMID 23349710.
. PMID 23349710. - ↑ Sawair, Faleh A.; Irwin, Christopher R.; Gordon, Derek J.; Leonard, Alan G.; Stephenson, Mike; Napier, Seamus S. (2003). "Invasive front grading: reliability and usefulness in the management of oral squamous cell carcinoma". Journal of Oral Pathology and Medicine. 32 (1): 1–9. doi:10.1034/j.1600-0714.2003.00060.x. PMID 12558952.
- ↑ Social inequalities in oral health: from evidence to action (PDF). 2015. p. 9. ISBN 9780952737766.
- ↑ The Oral Cancer Foundation
- ↑ "Oral cancer statistics". Cancer Research UK. Retrieved 28 October 2014.
- ↑ "Incidence & Prevalence of Oral cancer". Retrieved 14 May 2015.
External links
| Wikimedia Commons has media related to Oral cancer. |
- A digital manual for the early diagnosis of oral neoplasia (IARC Screening Group)
- Information about oral cancers from Stanford Hospital
- Information graphic about oral cancer and HPV link from Mount Sinai Hospital, New York
- The Oral Cancer Foundation