Octane
| | |
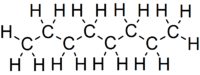 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Octane[1] | |
| Identifiers | |
| 111-65-9 | |
| 3D model (Jmol) | Interactive image |
| 3DMet | B00281 |
| 1696875 | |
| ChEBI | CHEBI:17590 |
| ChEMBL | ChEMBL134886 |
| ChemSpider | 349 |
| DrugBank | DB02440 |
| ECHA InfoCard | 100.003.539 |
| EC Number | 203-892-1 |
| 82412 | |
| KEGG | C01387 |
| MeSH | octane |
| PubChem | 356 |
| RTECS number | RG8400000 |
| UN number | 1262 |
| |
| |
| Properties | |
| C8H18 | |
| Molar mass | 114.23 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Gasoline-like[2] |
| Density | 0.703 g cm−3 |
| Melting point | −57.1 to −56.6 °C; −70.9 to −69.8 °F; 216.0 to 216.6 K |
| Boiling point | 125.1 to 126.1 °C; 257.1 to 258.9 °F; 398.2 to 399.2 K |
| 0.007 mg dm−3 (at 20 °C) | |
| log P | 4.783 |
| Vapor pressure | 1.47 kPa (at 20.0 °C) |
| Henry's law constant (kH) |
29 nmol Pa−1 kg−1 |
| Refractive index (nD) |
1.398 |
| Viscosity | 542 μPa s (at 20 °C) |
| Thermochemistry | |
| 255.68 J K−1 mol−1 | |
| Std molar entropy (S |
361.20 J K−1 mol−1 |
| Std enthalpy of formation (ΔfH |
−252.1–−248.5 kJ mol−1 |
| Std enthalpy of combustion (ΔcH |
−5.53–−5.33 MJ mol−1 |
| Hazards | |
| GHS pictograms |   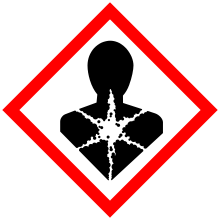 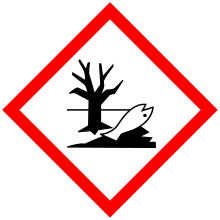 |
| GHS signal word | DANGER |
| H225, H304, H315, H336, H410 | |
| P210, P261, P273, P301+310, P331 | |
| EU classification (DSD) |
|
| R-phrases | R11, R38, R50/53, R65, R67 |
| S-phrases | (S2), S16, S29, S33 |
| NFPA 704 | |
| Flash point | 13.0 °C (55.4 °F; 286.1 K) |
| 220.0 °C (428.0 °F; 493.1 K) | |
| Explosive limits | 0.96–6.5% |
| Lethal dose or concentration (LD, LC): | |
| LDLo (lowest published) |
428 mg/kg (mouse, intravenous)[3] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 500 ppm (2350 mg/m3)[2] |
| REL (Recommended) |
TWA 75 ppm (350 mg/m3) C 385 ppm (1800 mg/m3) [15-minute][2] |
| IDLH (Immediate danger) |
1000 ppm[2] |
| Related compounds | |
| Related alkanes |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
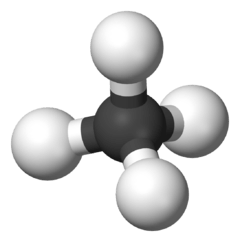
Octane is a hydrocarbon and an alkane with the chemical formula C8H18, and the condensed structural formula CH3(CH2)6CH3. Octane has many structural isomers that differ by the amount and location of branching in the carbon chain. One of these isomers, 2,2,4-trimethylpentane (isooctane) is used as one of the standard values in the octane rating scale.
Octane is a component of gasoline (petrol). As with all low-molecular-weight hydrocarbons, octane is volatile and very flammable.
Use of the term in gasoline
"Octane" is colloquially used as a short form of "octane rating" (an index of a fuel's ability to resist engine knock at high compression ratios, which is a characteristic of octane's branched-chain isomers, especially iso-octane), particularly in the expression "high octane."
The octane rating was originally determined by mixing a gasoline made entirely of heptane and 2,2,4-trimethylpentane (a highly branched octane), and assigning anti-knock ratings of 0 for pure heptane and 100 for pure 2,2,4-trimethylpentane. The anti-knock rating of this mixture would be the same as the percentage of 2,2,4-trimethylpentane in the mix. Modern octane ratings of gasoline are given octane ratings equal to those from this original heptane/octane scale. Different isomers of octane can contribute to a higher or lower octane rating. For example, n-octane (the straight chain of 8 carbon atoms with no branching) has a -10 (negative) octane rating, while pure 2,2,4-trimethylpentane (a highly branched octane) has an octane rating of 100.[4] Some fuels have an octane rating higher than 100, notably those containing methanol or ethanol.
Metaphorical use
Octane became well known in American popular culture in the mid- and late 1960s, when gasoline companies boasted of "high octane" levels in their gasoline advertisements.
The compound adjective "high-octane" , meaning powerful or dynamic, is recorded in a figurative sense from 1944.[5] By the mid-1990s, the phrase was commonly being used as an intensifier and has found a place in modern English vernacular.
Isomers
Octane has 18 structural isomers (24 including stereoisomers):
- Octane (n-octane)
- 2-Methylheptane
- 3-Methylheptane (2 enantiomers)
- 4-Methylheptane
- 3-Ethylhexane
- 2,2-Dimethylhexane
- 2,3-Dimethylhexane (2 enantiomers)
- 2,4-Dimethylhexane (2 enantiomers)
- 2,5-Dimethylhexane
- 3,3-Dimethylhexane
- 3,4-Dimethylhexane (2 enantiomers + 1 meso compound)
- 3-Ethyl-2-methylpentane
- 3-Ethyl-3-methylpentane
- 2,2,3-Trimethylpentane (2 enantiomers)
- 2,2,4-Trimethylpentane (isooctane)
- 2,3,3-Trimethylpentane
- 2,3,4-Trimethylpentane
- 2,2,3,3-Tetramethylbutane
References
- ↑ "octane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records. Retrieved 6 January 2012.
- 1 2 3 4 "NIOSH Pocket Guide to Chemical Hazards #0470". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "Octane". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ eejit's guides – Octane ratings explained
- ↑ Oxford English Dictionary.
External links
- International Chemical Safety Card 0933
- "NIOSH Pocket Guide to Chemical Hazards #0470". National Institute for Occupational Safety and Health (NIOSH).
- Dr. Duke's Phytochemical and Ethnobotanical Databases, Octane, http://www.ars-grin.gov/cgi-bin/duke/chemical.pl?OCTANE