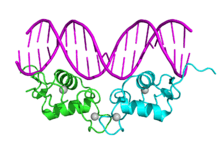
Nuclear receptor

In the field of molecular biology, nuclear receptors are a class of proteins found within cells that are responsible for sensing steroid and thyroid hormones and certain other molecules. In response, these receptors work with other proteins to regulate the expression of specific genes, thereby controlling the development, homeostasis, and metabolism of the organism.
Nuclear receptors have the ability to directly bind to DNA and regulate the expression of adjacent genes, hence these receptors are classified as transcription factors.[2][3] The regulation of gene expression by nuclear receptors generally only happens when a ligand — a molecule that affects the receptor's behavior — is present. More specifically, ligand binding to a nuclear receptor results in a conformational change in the receptor, which, in turn, activates the receptor, resulting in up- or down-regulation of gene expression.
A unique property of nuclear receptors that differentiates them from other classes of receptors is their ability to directly interact with and control the expression of genomic DNA. As a consequence, nuclear receptors play key roles in both embryonic development and adult homeostasis. As discussed below, nuclear receptors may be classified according to either mechanism[4][5] or homology.[6][7]
Species distribution
Nuclear receptors are specific to metazoans (animals) and are not found in protists, algae, fungi, or plants.[8] Amongst the early-branching animal lineages with sequenced genomes, two have been reported from the sponge Amphimedon queenslandica, two from the ctenophore Mnemiopsis leidyi[9] four from the placozoan Trichoplax adhaerens and 17 from the cnidarian Nematostella vectensis.[10] There are 270 nuclear receptors in the nematode C. elegans alone.[11] Humans, mice, and rats have respectively 48, 49, and 47 nuclear receptors each.[12]
Ligands

Ligands that bind to and activate nuclear receptors include lipophilic substances such as endogenous hormones, vitamins A and D, and xenobiotic endocrine disruptors. Because the expression of a large number of genes is regulated by nuclear receptors, ligands that activate these receptors can have profound effects on the organism. Many of these regulated genes are associated with various diseases, which explains why the molecular targets of approximately 13% of U.S. Food and Drug Administration (FDA) approved drugs target nuclear receptors.[13]
A number of nuclear receptors, referred to as orphan receptors,[14] have no known (or at least generally agreed upon) endogenous ligands. Some of these receptors such as FXR, LXR, and PPAR bind a number of metabolic intermediates such as fatty acids, bile acids and/or sterols with relatively low affinity. These receptors hence may function as metabolic sensors. Other nuclear receptors, such as CAR and PXR appear to function as xenobiotic sensors up-regulating the expression of cytochrome P450 enzymes that metabolize these xenobiotics.[15]
Structure
Most nuclear receptors have molecular masses between 50,000 and 100,000 daltons.
Nuclear receptors are modular in structure and contain the following domains:[16][17]
- (A-B) N-terminal regulatory domain: Contains the activation function 1 (AF-1) whose action is independent of the presence of ligand.[18] The transcriptional activation of AF-1 is normally very weak, but it does synergize with AF-2 in the E-domain (see below) to produce a more robust upregulation of gene expression. The A-B domain is highly variable in sequence between various nuclear receptors.
- (C) DNA-binding domain (DBD): Highly conserved domain containing two zinc fingers that binds to specific sequences of DNA called hormone response elements (HRE).
- (D) Hinge region: Thought to be a flexible domain that connects the DBD with the LBD. Influences intracellular trafficking and subcellular distribution.
- (E) Ligand binding domain (LBD): Moderately conserved in sequence and highly conserved in structure between the various nuclear receptors. The structure of the LBD is referred to as an alpha helical sandwich fold in which three anti parallel alpha helices (the "sandwich filling") are flanked by two alpha helices on one side and three on the other (the "bread"). The ligand binding cavity is within the interior of the LBD and just below three anti parallel alpha helical sandwich "filling". Along with the DBD, the LBD contributes to the dimerization interface of the receptor and in addition, binds coactivator and corepressor proteins. The LBD also contains the activation function 2 (AF-2) whose action is dependent on the presence of bound ligand.[18]
- (F) C-terminal domain: Highly variable in sequence between various nuclear receptors.
The N-terminal (A/B), DNA-binding (C), and ligand binding (E) domains are independently well folded and structurally stable while the hinge region (D) and optional C-terminal (F) domains may be conformationally flexible and disordered.[19] Domains relative orientations are very different by comparing three known multi-domain crystal structures, two of them binding on DR1,[1][20] one binding on DR4.[21]
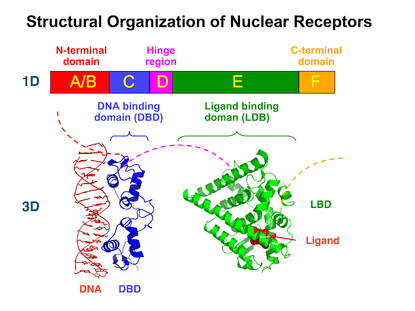 Structural Organization of Nuclear Receptors Top – Schematic 1D amino acid sequence of a nuclear receptor. Bottom – 3D structures of the DBD (bound to DNA) and LBD (bound to hormone) regions of the nuclear receptor. The structures shown are of the estrogen receptor. Experimental structures of N-terminal domain (A/B), hinge region (D), and C-terminal domain (F) have not been determined therefore are represented by red, purple, and orange dashed lines, respectively. |
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Mechanism of action
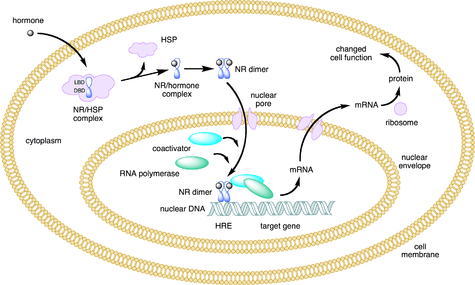

Nuclear receptors are multifunctional proteins that transduce signals of their cognate ligands. Nuclear receptors (NRs) may be classified into two broad classes according to their mechanism of action and subcellular distribution in the absence of ligand.
Small lipophilic substances such as natural hormones diffuse through the cell membrane and bind to nuclear receptors located in the cytosol (type I NR) or nucleus (type II NR) of the cell. Binding causes a conformational change in the receptor which, depending on the class of receptor, triggers a cascade of downstream events that direct the NRs to DNA transcription regulation sites which result in up or down-regulation of gene expression. In addition, two additional classes, type III which are a variant of type I, and type IV that bind DNA as monomers have also been identified.[4]
Accordingly, nuclear receptors may be subdivided into the following four mechanistic classes:[4][5]
Type I
Ligand binding to type I nuclear receptors in the cytosol results in the dissociation of heat shock proteins, homo-dimerization, translocation (i.e., active transport) from the cytoplasm into the cell nucleus, and binding to specific sequences of DNA known as hormone response elements (HREs). Type I nuclear receptors bind to HREs consisting of two half-sites separated by a variable length of DNA, and the second half-site has a sequence inverted from the first (inverted repeat). Type I nuclear receptors include members of subfamily 3, such as the androgen receptor, estrogen receptors, glucocorticoid receptor, and progesterone receptor.[24]
It has been noted that some of the NR subfamily 2 nuclear receptors may bind to direct repeat instead of inverted repeat HREs. In addition, some nuclear receptors that bind either as monomers or dimers, with only a single DNA binding domain of the receptor attaching to a single half site HRE. These nuclear receptors are considered orphan receptors, as their endogenous ligands are still unknown.
The nuclear receptor/DNA complex then recruits other proteins that transcribe DNA downstream from the HRE into messenger RNA and eventually protein, which causes a change in cell function.
Type II
Type II receptors, in contrast to type I, are retained in the nucleus regardless of the ligand binding status and in addition bind as hetero-dimers (usually with RXR) to DNA. In the absence of ligand, type II nuclear receptors are often complexed with corepressor proteins. Ligand binding to the nuclear receptor causes dissociation of corepressor and recruitment of coactivator proteins. Additional proteins including RNA polymerase are then recruited to the NR/DNA complex that transcribe DNA into messenger RNA.
Type II nuclear receptors include principally subfamily 1, for example the retinoic acid receptor, retinoid X receptor and thyroid hormone receptor.[25]
Type III
Type III nuclear receptors (principally NR subfamily 2) are similar to type I receptors in that both classes bind to DNA as homodimers. However, type III nuclear receptors, in contrast to type I, bind to direct repeat instead of inverted repeat HREs.
Type IV
Type IV nuclear receptors bind either as monomers or dimers, but only a single DNA binding domain of the receptor binds to a single half site HRE. Examples of type IV receptors are found in most of the NR subfamilies.
Coregulatory proteins
Nuclear receptors bound to hormone response elements recruit a significant number of other proteins (referred to as transcription coregulators) that facilitate or inhibit the transcription of the associated target gene into mRNA.[26][27] The function of these coregulators are varied and include chromatin remodeling (making the target gene either more or less accessible to transcription) or a bridging function to stabilize the binding of other coregulatory proteins. Nuclear receptors may bind specifically to a number of coregulator proteins, and thereby influence cellular mechanisms of signal transduction both directly, as well as indirectly.[28]
Coactivators
Binding of agonist ligands (see section below) to nuclear receptors induces a conformation of the receptor that preferentially binds coactivator proteins. These proteins often have an intrinsic histone acetyltransferase (HAT) activity, which weakens the association of histones to DNA, and therefore promotes gene transcription.
Corepressors
Binding of antagonist ligands to nuclear receptors in contrast induces a conformation of the receptor that preferentially binds corepressor proteins. These proteins, in turn, recruit histone deacetylases (HDACs), which strengthens the association of histones to DNA, and therefore represses gene transcription.
Agonism vs antagonism

Depending on the receptor involved, the chemical structure of the ligand and the tissue that is being affected, nuclear receptor ligands may display dramatically diverse effects ranging in a spectrum from agonism to antagonism to inverse agonism.[31]
Agonists
The activity of endogenous ligands (such as the hormones estradiol and testosterone) when bound to their cognate nuclear receptors is normally to upregulate gene expression. This stimulation of gene expression by the ligand is referred to as an agonist response. The agonistic effects of endogenous hormones can also be mimicked by certain synthetic ligands, for example, the glucocorticoid receptor anti-inflammatory drug dexamethasone. Agonist ligands work by inducing a conformation of the receptor which favors coactivator binding (see upper half of the figure to the right).
Antagonists
Other synthetic nuclear receptor ligands have no apparent effect on gene transcription in the absence of endogenous ligand. However they block the effect of agonist through competitive binding to the same binding site in the nuclear receptor. These ligands are referred to as antagonists. An example of antagonistic nuclear receptor drug is mifepristone which binds to the glucocorticoid and progesterone receptors and therefore blocks the activity of the endogenous hormones cortisol and progesterone respectively. Antagonist ligands work by inducing a conformation of the receptor which prevents coactivator and promotes corepressor binding (see lower half of the figure to the right).
Inverse agonists
Finally, some nuclear receptors promote a low level of gene transcription in the absence of agonists (also referred to as basal or constitutive activity). Synthetic ligands which reduce this basal level of activity in nuclear receptors are known as inverse agonists.[32]
Selective receptor modulators
A number of drugs that work through nuclear receptors display an agonist response in some tissues and an antagonistic response in other tissues. This behavior may have substantial benefits since it may allow retaining the desired beneficial therapeutic effects of a drug while minimizing undesirable side effects. Drugs with this mixed agonist/antagonist profile of action are referred to as selective receptor modulators (SRMs). Examples include Selective Androgen Receptor Modulators (SARMs), Selective Estrogen Receptor Modulators (SERMs) and Selective Progesterone Receptor Modulators (SPRMs). The mechanism of action of SRMs may vary depending on the chemical structure of the ligand and the receptor involved, however it is thought that many SRMs work by promoting a conformation of the receptor that is closely balanced between agonism and antagonism. In tissues where the concentration of coactivator proteins is higher than corepressors, the equilibrium is shifted in the agonist direction. Conversely in tissues where corepressors dominate, the ligand behaves as an antagonist.[33]
Alternative mechanisms

Transrepression
The most common mechanism of nuclear receptor action involves direct binding of the nuclear receptor to a DNA hormone response element. This mechanism is referred to as transactivation. However some nuclear receptors not only have the ability to directly bind to DNA, but also to other transcription factors. This binding often results in deactivation of the second transcription factor in a process known as transrepression.[34] One example of a nuclear receptor that are able to transrepress is the glucocorticoid receptor (GR). Furthermore, certain GR ligands known as Selective Glucocorticoid Receptor Agonists (SEGRAs) are able to activate GR in such a way that GR more strongly transrepresses than transactivates. This selectivity increases the separation between the desired antiinflammatory effects and undesired metabolic side effects of these selective glucocorticoids.
Non-genomic
The classical direct effects of nuclear receptors on gene regulation normally take hours before a functional effect is seen in cells because of the large number of intermediate steps between nuclear receptor activation and changes in protein expression levels. However it has been observed that many effects of the application of nuclear hormones, such as changes in ion channel activity, occur within minutes which is inconsistent with the classical mechanism of nuclear receptor action. While the molecular target for these non-genomic effects of nuclear receptors has not been conclusively demonstrated, it has been hypothesized that there are variants of nuclear receptors which are membrane associated instead of being localized in the cytosol or nucleus. Furthermore, these membrane associated receptors function through alternative signal transduction mechanisms not involving gene regulation.[35][36]
While it has been hypothesized that there are several membrane associated receptors for nuclear hormones, many of the rapid effects have been shown to require canonical nuclear receptors.[37][38] However, testing the relative importance of the genomic and nongenomic mechanisms in vivo has been prevented by the absence of specific molecular mechanisms for the nongenomic effects that could be blocked by mutation of the receptor without disrupting its direct effects on gene expression.
A molecular mechanism for non-genomic signaling through the nuclear thyroid hormone receptor TRβ involves the phosphatidylinositol 3-kinase (PI3K).[39] This signaling can be blocked by a single tyrosine to phenylalanine substitution in TRβ without disrupting direct gene regulation.[40] When mice were created with this single, conservative amino acid substitution in TRβ,[40] synaptic maturation and plasticity in the hippocampus was impaired almost as effectively as completely blocking thyroid hormone synthesis.[41] This mechanism appears to be conserved in all mammals but not in TRα or any other nuclear receptors. Thus, phosphotyrosine-dependent association of TRβ with PI3K provides a potential mechanism for integrating regulation of development and metabolism by thyroid hormone and receptor tyrosine kinases. In addition, thyroid hormone signaling through PI3K can alter gene expression.[42]
Family members
The following is a list of the 48 known human nuclear receptors plus selected non-human receptors[12] categorized according to sequence homology.[6][7]
| Subfamily | Group | Member | ||||||
|---|---|---|---|---|---|---|---|---|
| NRNC Symbol[6] | Abbreviation | Name | Gene | Ligand(s) | ||||
| 1 | Thyroid Hormone Receptor-like | A | Thyroid hormone receptor | NR1A1 | TRα | Thyroid hormone receptor-α | THRA | thyroid hormone |
| NR1A2 | TRβ | Thyroid hormone receptor-β | THRB | |||||
| B | Retinoic acid receptor | NR1B1 | RARα | Retinoic acid receptor-α | RARA | vitamin A and related compounds | ||
| NR1B2 | RARβ | Retinoic acid receptor-β | RARB | |||||
| NR1B3 | RARγ | Retinoic acid receptor-γ | RARG | |||||
| C | Peroxisome proliferator-activated receptor | NR1C1 | PPARα | Peroxisome proliferator-activated receptor-α | PPARA | fatty acids, prostaglandins | ||
| NR1C2 | PPAR-β/δ | Peroxisome proliferator-activated receptor-β/δ | PPARD | |||||
| NR1C3 | PPARγ | Peroxisome proliferator-activated receptor-γ | PPARG | |||||
| D | Rev-ErbA | NR1D1 | Rev-ErbAα | Rev-ErbAα | NR1D1 | heme | ||
| NR1D2 | Rev-ErbAβ | Rev-ErbAα | NR1D2 | |||||
| F | RAR-related orphan receptor | NR1F1 | RORα | RAR-related orphan receptor-α | RORA | cholesterol, ATRA | ||
| NR1F2 | RORβ | RAR-related orphan receptor-β | RORB | |||||
| NR1F3 | RORγ | RAR-related orphan receptor-γ | RORC | |||||
| H | Liver X receptor-like | NR1H3 | LXRα | Liver X receptor-α | NR1H3 | oxysterols | ||
| NR1H2 | LXRβ | Liver X receptor-β | NR1H2 | |||||
| NR1H4 | FXR | Farnesoid X receptor | NR1H4 | |||||
| NR1H5[43] | FXR-β | Farnesoid X receptor-β | NR1H5P | |||||
| I | Vitamin D receptor-like | NR1I1 | VDR | Vitamin D receptor | VDR | vitamin D | ||
| NR1I2 | PXR | Pregnane X receptor | NR1I2 | xenobiotics | ||||
| NR1I3 | CAR | Constitutive androstane receptor | NR1I3 | androstane | ||||
| X | NRs with two DNA binding domains[44][45] | NR1X1 | 2DBD-NRα | |||||
| NR1X2 | 2DBD-NRβ | |||||||
| NR1X3 | 2DBD-NRγ | |||||||
| 2 | Retinoid X Receptor-like | A | Hepatocyte nuclear factor-4 | NR2A1 | HNF4α | Hepatocyte nuclear factor-4-α | HNF4A | fatty acids |
| NR2A2 | HNF4γ | Hepatocyte nuclear factor-4-γ | HNF4G | |||||
| B | Retinoid X receptor | NR2B1 | RXRα | Retinoid X receptor-α | RXRA | retinoids | ||
| NR2B2 | RXRβ | Retinoid X receptor-β | RXRB | |||||
| NR2B3 | RXRγ | Retinoid X receptor-γ | RXRG | |||||
| C | Testicular receptor | NR2C1 | TR2 | Testicular receptor 2 | NR2C1 | |||
| NR2C2 | TR4 | Testicular receptor 4 | NR2C2 | |||||
| E | TLX/PNR | NR2E1 | TLX | Homologue of the Drosophila tailless gene | NR2E1 | |||
| NR2E3 | PNR | Photoreceptor cell-specific nuclear receptor | NR2E3 | |||||
| F | COUP/EAR | NR2F1 | COUP-TFI | Chicken ovalbumin upstream promoter-transcription factor I | NR2F1 | |||
| NR2F2 | COUP-TFII | Chicken ovalbumin upstream promoter-transcription factor II | NR2F2 | |||||
| NR2F6 | EAR-2 | V-erbA-related | NR2F6 | |||||
| 3 | Estrogen Receptor-like | A | Estrogen receptor | NR3A1 | ERα | Estrogen receptor-α | ESR1 | estrogens |
| NR3A2 | ERβ | Estrogen receptor-β | ESR2 | |||||
| B | Estrogen related receptor | NR3B1 | ERRα | Estrogen-related receptor-α | ESRRA | |||
| NR3B2 | ERRβ | Estrogen-related receptor-β | ESRRB | |||||
| NR3B3 | ERRγ | Estrogen-related receptor-γ | ESRRG | |||||
| C | 3-Ketosteroid receptors | NR3C1 | GR | Glucocorticoid receptor | NR3C1 | cortisol | ||
| NR3C2 | MR | Mineralocorticoid receptor | NR3C2 | aldosterone | ||||
| NR3C3 | PR | Progesterone receptor | PGR | progesterone | ||||
| NR3C4 | AR | Androgen receptor | AR | testosterone | ||||
| 4 | Nerve Growth Factor IB-like | A | NGFIB/NURR1/NOR1 | NR4A1 | NGFIB | Nerve Growth factor IB | NR4A1 | |
| NR4A2 | NURR1 | Nuclear receptor related 1 | NR4A2 | |||||
| NR4A3 | NOR1 | Neuron-derived orphan receptor 1 | NR4A3 | |||||
| 5 | Steroidogenic Factor-like | A | SF1/LRH1 | NR5A1 | SF1 | Steroidogenic factor 1 | NR5A1 | phosphatidylinositols |
| NR5A2 | LRH-1 | Liver receptor homolog-1 | NR5A2 | phosphatidylinositols | ||||
| 6 | Germ Cell Nuclear Factor-like | A | GCNF | NR6A1 | GCNF | Germ cell nuclear factor | NR6A1 | |
| 0 | Miscellaneous | B | DAX/SHP | NR0B1 | DAX1 | Dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1 | NR0B1 | |
| NR0B2 | SHP | Small heterodimer partner | NR0B2 | |||||
History
Below is a brief selection of key events in the history of nuclear receptor research.[46]
- 1905 – Ernest Starling coined the word hormone
- 1926 – Edward Calvin Kendall and Tadeus Reichstein isolated and determined the structures of cortisone and thyroxine
- 1929 – Adolf Butenandt and Edward Adelbert Doisy – independently isolated and determined the structure of estrogen
- 1958 – Elwood Jensen – isolated the estrogen receptor
- 1980s – cloning of the estrogen, glucocorticoid, and thyroid hormone receptors by Pierre Chambon, Ronald Evans, and Björn Vennström respectively
- 2004 – Pierre Chambon, Ronald Evans, and Elwood Jensen were awarded the Albert Lasker Award for Basic Medical Research, an award that frequently precedes a Nobel Prize in Medicine
See also
References
- 1 2 PDB: 3E00; Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, Rastinejad F (October 2008). "Structure of the intact PPAR-gamma-RXR-alpha nuclear receptor complex on DNA". Nature. 456 (7220): 350–356. doi:10.1038/nature07413. PMC 2743566
 . PMID 19043829.
. PMID 19043829. - ↑ Evans RM (1988). "The steroid and thyroid hormone receptor superfamily". Science. 240 (4854): 889–95. doi:10.1126/science.3283939. PMID 3283939.
- ↑ Olefsky JM (2001). "Nuclear receptor minireview series". J. Biol. Chem. 276 (40): 36863–4. doi:10.1074/jbc.R100047200. PMID 11459855.
- 1 2 3 Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM (1995). "The nuclear receptor superfamily: the second decade". Cell. 83 (6): 835–9. doi:10.1016/0092-8674(95)90199-X. PMID 8521507.
- 1 2 Novac N, Heinzel T (2004). "Nuclear receptors: overview and classification". Curr Drug Targets Inflamm Allergy. 3 (4): 335–46. doi:10.2174/1568010042634541. PMID 15584884.
- 1 2 3 Nuclear Receptors Nomenclature Committee (1999). "A unified nomenclature system for the nuclear receptor superfamily". Cell. 97 (2): 161–3. doi:10.1016/S0092-8674(00)80726-6. PMID 10219237.
- 1 2 Laudet V (1997). "Evolution of the nuclear receptor superfamily: early diversification from an ancestral orphan receptor". J. Mol. Endocrinol. 19 (3): 207–26. doi:10.1677/jme.0.0190207. PMID 9460643.
- ↑ Escriva H, Langlois MC, Mendonça RL, Pierce R, Laudet V (May 1998). "Evolution and diversification of the nuclear receptor superfamily". Annals of the New York Academy of Sciences. 839: 143–6. doi:10.1111/j.1749-6632.1998.tb10747.x. PMID 9629140.
- ↑ Reitzel AM, Pang K, Ryan JF, Mullikin JC, Martindale MQ, Baxevanis AD, Tarrant AM (2011). "Nuclear receptors from the ctenophore Mnemiopsis leidyi lack a zinc-finger DNA-binding domain: lineage-specific loss or ancestral condition in the emergence of the nuclear receptor superfamily?". Evodevo. 2 (1): 3. doi:10.1186/2041-9139-2-3. PMC 3038971
 . PMID 21291545.
. PMID 21291545. - ↑ Bridgham JT, Eick GN, Larroux C, Deshpande K, Harms MJ, Gauthier ME, Ortlund EA, Degnan BM, Thornton JW (2010). "Protein evolution by molecular tinkering: diversification of the nuclear receptor superfamily from a ligand-dependent ancestor". PLoS Biol. 8 (10): e1000497. doi:10.1371/journal.pbio.1000497. PMC 2950128
 . PMID 20957188.
. PMID 20957188. - ↑ Sluder AE, Maina CV (April 2001). "Nuclear receptors in nematodes: themes and variations". Trends in Genetics : TIG. 17 (4): 206–13. doi:10.1016/S0168-9525(01)02242-9. PMID 11275326.
- 1 2 Zhang Z, Burch PE, Cooney AJ, Lanz RB, Pereira FA, Wu J, Gibbs RA, Weinstock G, Wheeler DA (2004). "Genomic analysis of the nuclear receptor family: new insights into structure, regulation, and evolution from the rat genome". Genome Res. 14 (4): 580–90. doi:10.1101/gr.2160004. PMC 383302
 . PMID 15059999.
. PMID 15059999. - ↑ Overington JP, Al-Lazikani B, Hopkins AL (2006). "How many drug targets are there?". Nature reviews. Drug discovery. 5 (12): 993–6. doi:10.1038/nrd2199. PMID 17139284.
- ↑ Benoit G, Cooney A, Giguere V, Ingraham H, Lazar M, Muscat G, Perlmann T, Renaud JP, Schwabe J, Sladek F, Tsai MJ, Laudet V (2006). "International Union of Pharmacology. LXVI. Orphan nuclear receptors". Pharmacol. Rev. 58 (4): 798–836. doi:10.1124/pr.58.4.10. PMID 17132856.
- ↑ Mohan R, Heyman RA (2003). "Orphan nuclear receptor modulators". Curr Top Med Chem. 3 (14): 1637–47. doi:10.2174/1568026033451709. PMID 14683519.
- ↑ Kumar R, Thompson EB (1999). "The structure of the nuclear hormone receptors". Steroids. 64 (5): 310–9. doi:10.1016/S0039-128X(99)00014-8. PMID 10406480.
- ↑ Klinge CM (2000). "Estrogen receptor interaction with co-activators and co-repressors". Steroids. 65 (5): 227–51. doi:10.1016/S0039-128X(99)00107-5. PMID 10751636.
- 1 2 Wärnmark A, Treuter E, Wright AP, Gustafsson J (2003). "Activation functions 1 and 2 of nuclear receptors: molecular strategies for transcriptional activation". Mol. Endocrinol. 17 (10): 1901–9. doi:10.1210/me.2002-0384. PMID 12893880.
- ↑ Weatherman RV, Fletterick RJ, Scanlan TS (1999). "Nuclear-receptor ligands and ligand-binding domains". Annu. Rev. Biochem. 68: 559–81. doi:10.1146/annurev.biochem.68.1.559. PMID 10872460.
- ↑ Chandra V, Huang P, Potluri N, Wu D, Kim Y, Rastinejad F (March 2013). "Multidomain integration in the structure of the HNF-4α nuclear receptor complex". Nature. 495 (7441): 394–398. doi:10.1038/nature11966. PMID 23485969.
- ↑ Lou X, Toresson G, Benod C, Suh JH, Philips KJ, Webb P, Gustafsson JA (February 2014). "Structure of the retinoid X receptor α-liver X receptor β (RXRα-LXRβ) heterodimer on DNA". Nat. Struct. Mol. Biol. 21 (3): 277–281. doi:10.1038/nsmb.2778. PMID 24561505.
- ↑ PDB: 2C7A; Roemer SC, Donham DC, Sherman L, Pon VH, Edwards DP, Churchill ME (December 2006). "Structure of the progesterone receptor-deoxyribonucleic acid complex: novel interactions required for binding to half-site response elements". Mol. Endocrinol. 20 (12): 3042–52. doi:10.1210/me.2005-0511. PMC 2532839
 . PMID 16931575.
. PMID 16931575. - ↑ PDB: 3L0L; Jin L, Martynowski D, Zheng S, Wada T, Xie W, Li Y (May 2010). "Structural basis for hydroxycholesterols as natural ligands of orphan nuclear receptor RORgamma". Mol. Endocrinol. 24 (5): 923–9. doi:10.1210/me.2009-0507. PMC 2870936
 . PMID 20203100.
. PMID 20203100. - ↑ Linja MJ, Porkka KP, Kang Z, Savinainen KJ, Jänne OA, Tammela TL, Vessella RL, Palvimo JJ, Visakorpi T (February 2004). "Expression of androgen receptor coregulators in prostate cancer". Clin. Cancer Res. 10 (3): 1032–40. doi:10.1158/1078-0432.CCR-0990-3. PMID 14871982.
- ↑ Klinge CM, Bodenner DL, Desai D, Niles RM, Traish AM (May 1997). "Binding of type II nuclear receptors and estrogen receptor to full and half-site estrogen response elements in vitro". Nucleic Acids Res. 25 (10): 1903–12. doi:10.1093/nar/25.10.1903. PMC 146682
 . PMID 9115356.
. PMID 9115356. - ↑ Glass CK, Rosenfeld MG (2000). "The coregulator exchange in transcriptional functions of nuclear receptors". Genes Dev. 14 (2): 121–41. doi:10.1101/gad.14.2.121. PMID 10652267.
- ↑ Aranda A, Pascual A (2001). "Nuclear hormone receptors and gene expression" (abstract). Physiol. Rev. 81 (3): 1269–304. PMID 11427696.
- ↑ Copland JA, Sheffield-Moore M, Koldzic-Zivanovic N, Gentry S, Lamprou G, Tzortzatou-Stathopoulou F, Zoumpourlis V, Urban RJ, Vlahopoulos SA (June 2009). "Sex steroid receptors in skeletal differentiation and epithelial neoplasia: is tissue-specific intervention possible?". BioEssays. 31 (6): 629–41. doi:10.1002/bies.200800138. PMID 19382224.
- ↑ Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engström O, Öhman L, Greene GL, Gustafsson J, Carlquist M (1997). "Molecular basis of agonism and antagonism in the oestrogen receptor". Nature. 389 (6652): 753–8. doi:10.1038/39645. PMID 9338790.
- ↑ Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL (1998). "The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen". Cell. 95 (7): 927–37. doi:10.1016/S0092-8674(00)81717-1. PMID 9875847.
- ↑ Gronemeyer H, Gustafsson JA, Laudet V (2004). "Principles for modulation of the nuclear receptor superfamily". Nature reviews. Drug discovery. 3 (11): 950–64. doi:10.1038/nrd1551. PMID 15520817.
- ↑ Busch BB, Stevens WC, Martin R, Ordentlich P, Zhou S, Sapp DW, Horlick RA, Mohan R (2004). "Identification of a selective inverse agonist for the orphan nuclear receptor estrogen-related receptor alpha". J. Med. Chem. 47 (23): 5593–6. doi:10.1021/jm049334f. PMID 15509154.
- ↑ Smith CL, O'Malley BW (2004). "Coregulator function: a key to understanding tissue specificity of selective receptor modulators". Endocr Rev. 25 (1): 45–71. doi:10.1210/er.2003-0023. PMID 14769827.
- ↑ Pascual G, Glass CK (2006). "Nuclear receptors versus inflammation: mechanisms of transrepression". Trends Endocrinol Metab. 17 (8): 321–7. doi:10.1016/j.tem.2006.08.005. PMID 16942889.
- ↑ Björnström L, Sjöberg M (2004). "Estrogen receptor-dependent activation of AP-1 via non-genomic signalling". Nucl Recept. 2 (1): 3. doi:10.1186/1478-1336-2-3. PMC 434532
 . PMID 15196329.
. PMID 15196329. - ↑ Zivadinovic D, Gametchu B, Watson CS (2005). "Membrane estrogen receptor-alpha levels in MCF-7 breast cancer cells predict cAMP and proliferation responses". Breast Cancer Res. 7 (1): R101–12. doi:10.1186/bcr958. PMC 1064104
 . PMID 15642158.
. PMID 15642158. - ↑ Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC (2001). "Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity". Cell. 104 (5): 719–30. doi:10.1016/S0092-8674(01)00268-9. PMID 11257226.
- ↑ Storey NM, Gentile S, Ullah H, Russo A, Muessel M, Erxleben C, Armstrong DL (2006). "Rapid signaling at the plasma membrane by a nuclear receptor for thyroid hormone". Proc. Natl. Acad. Sci. U.S.A. 103 (13): 5197–201. doi:10.1073/pnas.0600089103. PMC 1458817
 . PMID 16549781.
. PMID 16549781. - ↑ Storey NM, O'Bryan JP, Armstrong DL (2002). "Rac and Rho Mediate Opposing Hormonal Regulation of the Ether-A-Go-Go-Related Potassium Channel". Current Biology. 12 (1): 27–33. doi:10.1016/S0960-9822(01)00625-X. PMID 11790300.
- 1 2 Martin NP, Marron Fernandez de Velasco E, Mizuno F, Scappini EL, Gloss B, Erxleben C, Williams JG, Stapleton HM, Gentile S, Armstrong DL (2014). "A rapid cytoplasmic mechanism for PI3 kinase regulation by the nuclear thyroid hormone receptor, TRβ, and genetic evidence for its role in the maturation of mouse hippocampal synapses in vivo". Endocrinology. 155 (9): 3713–24. doi:10.1210/en.2013-2058. PMID 24932806.
- ↑ Gilbert ME (2004). "Alterations in synaptic transmission and plasticity in area CA1 of adult hippocampus following developmental hypothyroidism.". Brain Res Dev Brain Res. 148 (1): 11–18. doi:10.1016/j.devbrainres.2003.09.018. PMID 14757514.
- ↑ Moeller LC, Broecker-Preuss M (2011). "Transcriptional regulation by nonclassical action of thyroid hormone". Thyroid Res. 4 Suppl 1: S6. doi:10.1186/1756-6614-4-S1-S6. PMC 3155112
 . PMID 21835053.
. PMID 21835053. - ↑ Otte K, Kranz H, Kober I, Thompson P, Hoefer M, Haubold B, Remmel B, Voss H, Kaiser C, Albers M, Cheruvallath Z, Jackson D, Casari G, Koegl M, Pääbo S, Mous J, Kremoser C, Deuschle U (2003). "Identification of farnesoid X receptor beta as a novel mammalian nuclear receptor sensing lanosterol". Mol. Cell. Biol. 23 (3): 864–72. doi:10.1128/mcb.23.3.864-872.2003. PMC 140718
 . PMID 12529392.
. PMID 12529392. - ↑ Wu W, Niles EG, El-Sayed N, Berriman M, LoVerde PT (2006). "Schistosoma mansoni (Platyhelminthes, Trematoda) nuclear receptors: sixteen new members and a novel subfamily". Gene. 366 (2): 303–15. doi:10.1016/j.gene.2005.09.013. PMID 16406405.
- ↑ Wu W, Niles EG, Hirai H, LoVerde PT (2007). "Evolution of a novel subfamily of nuclear receptors with members that each contain two DNA binding domains". BMC Evol Biol. 7: 27. doi:10.1186/1471-2148-7-27. PMC 1810520
 . PMID 17319953.
. PMID 17319953. - ↑ Tata JR (2005). "One hundred years of hormones". EMBO Rep. 6 (6): 490–6. doi:10.1038/sj.embor.7400444. PMC 1369102
 . PMID 15940278.
. PMID 15940278.
External links
- Nuclear Receptors at the US National Library of Medicine Medical Subject Headings (MeSH)
- Vincent Laudet (2006). "The IUPHAR Compendium of the Pharmacology and Classification of the Nuclear Receptor Superfamily 2006E". Nuclear Receptor Compendium. The International Union of Basic and Clinical Pharmacology. Retrieved 2008-02-21.
- "Nuclear Receptor online journal". Home page. published by BioMed Central (no longer accepting submissions since May 2007). Retrieved 2008-02-21.
- "Nuclear Receptor Resource". Georgetown University. Retrieved 2008-02-21.
- "Nuclear Receptor Signaling Atlas (Receptors, Coactivators, Corepressors and Ligands)". The NURSA Consortium. Retrieved 2008-02-21.
an NIH-funded research consortium and database; includes open-access PubMed-indexed journal, Nuclear Receptor Signaling
- "Nuclear Receptor Resource". Jack Vanden Heuvel. Retrieved 2009-09-21.