Norleucine
 | |
| Names | |
|---|---|
| IUPAC name
(2S)-2-Aminohexanoic acid | |
| Other names
Caprine Glycoleucine | |
| Identifiers | |
| 327-57-1 (2S) | |
| 3D model (Jmol) | Interactive image |
| 3DMet | B00369 |
| 1721748 | |
| ChEBI | CHEBI:36405 |
| ChemSpider | 401917 |
| DrugBank | DB04419 |
| ECHA InfoCard | 100.005.748 |
| EC Number | 210-462-7 |
| 464584 | |
| KEGG | C01933 |
| MeSH | Norleucine |
| PubChem | 9475 |
| RTECS number | RC6308000 |
| UNII | 832C8OV84S |
| |
| |
| Properties | |
| C6H13NO2 | |
| Molar mass | 131.18 g·mol−1 |
| Melting point | 301 °C (574 °F; 574 K) (decomposes) [1] |
| 16 g/l at 23 °C [2] | |
| Acidity (pKa) | 2.39 (carboxyl), 9.76 (amino)[3] |
| Hazards | |
| R-phrases | 43 |
| S-phrases | S36/37 |
| Related compounds | |
| Related Aminoacids |
Norvaline (2-amino-pentanoic) Aminocaproic acid (6-amino-hexanoic) Leucine (2-amino-4-methyl-pentanoic) Isoleucine (2-amino-3-methyl-pentanoic) Lysine (2,6-diamino-hexanoic) |
| Related compounds |
Caproic acid (hexanoic) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
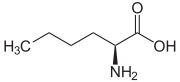
Norleucine (abbreviated as Nle) is an amino acid with the formula CH3(CH2)3CH(NH2)CO2H. A systematic name for this compound is 2-aminohexanoic acid.[4] The compound is an isomer of the more common amino acid leucine. Like most other α-amino acids, norleucine is chiral. It is a white, water-soluble solid.
Occurrence
Together with norvaline, norleucine is found in small amounts in some bacterial strains where its concentration can approach millimolar. Its biosynthesis has been examined. It arise via the action of 2-isopropylmalate synthase on α-ketobutyrate. The incorporation of Nle into peptides reflects the imperfect selectivity of the associated aminoacyl-tRNA synthetase. In Miller–Urey experiments probing prebiotic synthesis of amino acids, norleucine and especially norvaline are formed.[5]
Uses
It is nearly isosteric with methionine, even though it does not contain sulfur.[6] For this reason, norleucine has been used to probe the role of methionine in Amyloid-β peptide (AβP) the central constituent of senile plaques in Alzheimer's disease. A study showed that with the substitution of the methionine at the 35 position with norleucine the neurotoxic effects of the Aβ peptides were completely negated.[7]
See also
- Leucines, description of the isomers of leucine
- norvaline, isomer of valine that has similar biochemistry to that of norleucine.
References
- ↑ Hermann Römpp, Jürgen Falbe und Manfred Regitz: Römpp Lexikon Chemie, 9. Auflage, Georg Thieme Verlag, Stuttgart 1992.
- ↑ Sicherheitsdatenblatt Acros.
- ↑ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- ↑ The use of the name norleucine is discouraged as it is a misnomer, given than nor is defined for an amino acid with one less methylene group than found in the proteinogenic form. "Nomenclature and Symbolism For Amino Acids and Peptides". Pure and Applied Chemistry. 56 (5): 595–624. 1984. doi:10.1351/pac198456050595.
- ↑ Alvarez-Carreno, Claudia; Becerra, Arturo; Lazcano, Antonio "Norvaline and Norleucine May Have Been More Abundant Protein Components during Early Stages of Cell Evolution" Origins of Life and Evolution of Biospheres 2014, volume 43, 363-375. doi:10.1007/s11084-013-9344-3
- ↑ Moroder, Luis "Isosteric replacement of sulfur with other chalcogens in peptides and proteins" Journal of Peptide Science 2005, volume 11, 187-214. doi:10.1002/psc.654
- ↑ Clementi, ME & Misiti, F (Nov 2005). "Substitution of methionine 35 inhibits apoptotic effects of Abeta(31-35) and Abeta(25-35) fragments of amyloid-beta protein in PC12 cells". Med Sci Monit. 11 (11): BR381–5. PMID 16258386.