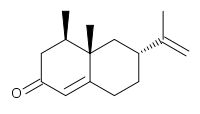
Nootkatone
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4-α,5-Dimethyl-1,2,3,4,4α,5,6,7-octahydro-7-keto-3-isopropenylnaphthalene | |
| Other names
(+)-nootkatone | |
| Identifiers | |
| 4674-50-4 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:81377 |
| ChEMBL | ChEMBL446299 |
| ChemSpider | 1064812 |
| ECHA InfoCard | 100.022.840 |
| KEGG | C17914 |
| PubChem | 1268142 |
| |
| |
| Properties | |
| C15H22O | |
| Molar mass | 218.34 g·mol−1 |
| Appearance | Clear or white crystals, impure samples appear as a viscous yellow liquid |
| Density | 0.968 g/mL |
| Melting point | 36 °C (97 °F; 309 K) |
| Boiling point | 170 °C (338 °F; 443 K) |
| Insoluble in water, very soluble in ethanol, dichloromethane, ethyl acetate, soluble in hexanes | |
| Hazards | |
| S-phrases | S23 S24 S25 |
| Flash point | ~ 100 °C (212 °F; 373 K) |
| Related compounds | |
| Related terpenes |
Valencene |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Nootkatone is a natural organic compound and is the most important and expensive aromatic of grapefruit.[1] It is a sesquiterpene and a ketone.
Nootkatone was previously thought to be one of the main chemical components of the smell and flavour of grapefruits. In its solid form it is usually found as crystals. As a liquid, it is viscous and yellow. Nootkatone is typically extracted from grapefruit, but can also be manufactured with genetically modified organisms, or through the chemical or biochemical oxidation of valencene. It is also found in Alaska yellow cedar trees[2] and vetiver grass.[3]
Uses
Nootkatone in spray form has been shown as an effective repellent/insecticide against deer ticks[3][4][5] and lone star ticks.[4][5] It is also an effective repellent/insecticide against mosquitos, and may repel bed bugs, head lice and other insects.[6] It is an environmentally friendly insecticide, because it is a volatile essential oil that does not persist in the environment.[6] It is nontoxic to humans, is an approved food additive,[6] and "is commonly used in foods, cosmetics, and pharmaceuticals".[3]
The CDC has licensed patents to two companies to produce an insecticide and an insect repellant.[6] Allylix, of San Diego, CA (Now Evolva), is one of these licensees [7] and has developed an enzyme fermentation process that will produce nookatone more cost effectively.[8]
See also
References
- ↑ Furusawa, Mai; Toshihiro Hashimoto; Yoshiaki Noma; Yoshinori Asakawa (November 2005). "Highly Efficient Production of Nootkatone, the Grapefruit Aroma from Valencene, by Biotransformation". Chem. Pharm. Bull. 53 (11): 1513–1514. doi:10.1248/cpb.53.1513. PMID 16272746.
- ↑ Panella, NA.; Dolan, MC.; Karchesy, JJ.; Xiong, Y.; Peralta-Cruz, J.; Khasawneh, M.; Montenieri, JA.; Maupin, GO. (May 2005). "Use of novel compounds for pest control: insecticidal and acaricidal activity of essential oil components from heartwood of Alaska yellow cedar.". J Med Entomol. 42 (3): 352–8. doi:10.1603/0022-2585(2005)042[0352:UONCFP]2.0.CO;2. PMID 15962787.
- 1 2 3 Jan Suszkiw (January 2011). "Lignin + Nootkatone = Dead Ticks". USDA.
- 1 2 Dolan, MC.; Jordan, RA.; Schulze, TL.; Schulze, CJ.; Manning, MC.; Ruffolo, D.; Schmidt, JP.; Piesman, J.; Karchesy, JJ. (Dec 2009). "Ability of two natural products, nootkatone and carvacrol, to suppress Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in a Lyme disease endemic area of New Jersey". J Econ Entomol. 102 (6): 2316–24. doi:10.1603/029.102.0638. PMID 20069863.
- 1 2 Jordan, Robert A.; Schulze, Terry L.; Dolan, Marc C. (January 2012). "Efficacy of Plant-Derived and Synthetic Compounds on Clothing as Repellents Against Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae)". Journal of Medical Entomology. 49 (1): 101–106. doi:10.1603/ME10241. PMID 22308777.
- 1 2 3 4 Richard Knox (April 18, 2011). "Repelling Bugs With The Essence Of Grapefruit". NPR.
- ↑ Bigelow, Bruce (2011-04-28). "Nootkatone, So A-peeling in Grapefruit, is Repellent to Mosquitoes and Ticks". xconomy.com. Retrieved 10 August 2012.
- ↑ "Cost effective fermentation replaces costly exration". Allylix. Retrieved 10 August 2012.