Non-small-cell lung carcinoma
| Non-small-cell lung carcinoma | |
|---|---|
 | |
| Micrograph of a squamous carcinoma, a type of non-small-cell lung carcinoma. FNA specimen. Pap stain. | |
| Classification and external resources | |
| ICD-9-CM | 162 |
| MedlinePlus | 007194 |
| eMedicine | med/1333 |
| MeSH | D002289 |
Non-small-cell lung carcinoma (NSCLC) is any type of epithelial lung cancer other than small cell lung carcinoma (SCLC). NSCLC accounts for about 85% of all lung cancers.[1][2] As a class, NSCLCs are relatively insensitive to chemotherapy, compared to small cell carcinoma. When possible, they are primarily treated by surgical resection with curative intent, although chemotherapy is increasingly being used both pre-operatively (neoadjuvant chemotherapy) and post-operatively (adjuvant chemotherapy).
Types
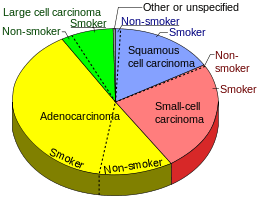
The most common types of NSCLC are squamous cell carcinoma, large cell carcinoma, and adenocarcinoma, but there are several other types that occur less frequently, and all types can occur in unusual histologic variants and as mixed cell-type combinations.[4] Non-squamous cell carcinoma almost occupy the half of NSCLC. In the tissue classification, the centural type contains about one-ninth.
Sometimes the phrase "not otherwise specified", or NOS is used generically, usually when a more specific diagnosis cannot be made. This is most often the case when a pathologist examines a small amount of malignant cells or tissue in a cytology or biopsy specimen.[4]
Lung cancer in never-smokers is almost universally NSCLC, with a sizeable majority being adenocarcinoma.[5]
On relatively rare occasions, malignant lung tumors are found to contain components of both SCLC and NSCLC. In these cases, the tumors should be classified as combined small cell lung carcinoma (c-SCLC),[6] and are (usually) treated like "pure" SCLC.[7]
Lung adenocarcinoma
Adenocarcinoma of the lung is currently the most common type of lung cancer in "never smokers" (lifelong non-smokers).[8] Adenocarcinomas account for approximately 40% of lung cancers. Historically, adenocarcinoma was more often seen peripherally in the lungs than small cell lung cancer and squamous cell lung cancer, both of which tended to be more often centrally located.[9][10] However, recent studies suggest that the "ratio of centrally-to-peripherally occurring" lesions may be converging toward unity for both adenocarcinoma and squamous cell carcinoma.
Squamous cell lung carcinoma

Squamous cell carcinoma (SCC) of the lung is more common in men than in women. It is closely correlated with a history of tobacco smoking, more so than most other types of lung cancer. According to the Nurses' Health Study, the relative risk of SCC is approximately 5.5, both among those with a previous duration of smoking of 1 to 20 years, and those with 20 to 30 years, compared to never-smokers.[11] The relative risk increases to approximately 16 with a previous smoking duration of 30 to 40 years, and approximately 22 with more than 40 years.[11]
Large-cell lung carcinoma
Large cell lung carcinoma (LCLC) is a heterogeneous group of undifferentiated malignant neoplasms originating from transformed epithelial cells in the lung. LCLC's have typically comprised around 10% of all NSCLC in the past, although newer diagnostic techniques seem to be reducing the incidence of diagnosis of "classic" LCLC in favor of more poorly differentiated squamous cell carcinomas and adenocarcinomas.[12] LCLC is, in effect, a "diagnosis of exclusion", in that the tumor cells lack light microscopic characteristics that would classify the neoplasm as a small-cell carcinoma, squamous-cell carcinoma, adenocarcinoma, or other more specific histologic type of lung cancer. LCLC is differentiated from small cell lung carcinoma (SCLC) primarily by the larger size of the anaplastic cells, a higher cytoplasmic-to-nuclear size ratio, and a lack of "salt-and-pepper" chromatin.
Staging
Staging is a formal procedure to determine how developed the cancer is. This determines treatment options.
The American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) recommend TNM staging, using a uniform scheme for non-small cell lung carcinoma, small-cell lung carcinoma and broncho-pulmonary carcinoid tumors.[13] AJCC has provided an accessible poster version of these copyrighted TNM descriptors to which readers are directed.[13] Extensive presentation of prognostic data for both 6th and 7th edition individual TNM descriptors and overall groups is available.[14]
There are several components of NSCLC staging which then influence physicians' treatment strategies.[15] The lung tumor itself is typically assessed both radiographically for overall size as well as by a pathologist under the microscope to identify specific genetic markers or to see if there has been invasion into important structures within the chest (e.g., bronchus or pleural cavity). Next, the patient's nearby lymph nodes within the chest cavity known as the mediastinum will be checked for disease involvement. Finally, the patient will be evaluated for more distant sites of metastatic disease, most typically with brain imaging and or scans of the bones.
Treatment
More than one kind of treatment is often used, depending on the stage of the cancer, the individual's overall health, age, response to chemotherapy, and other factors such as the likely side effects of the treatment. After full staging, the NSCLC patient can typically be classified in one of three different categories: patients with early, non-metastatic disease (Stage I, II and select III tumors), patients with locally advanced disease confined to the thoracic cavity (e.g., large tumors, tumors involving critical chest structures or patients with positive mediastinal lymph nodes) or patients with distant metastasis outside of the thoracic cavity.
Early/non-metastatic NSCLC
NSCLCs are usually not very sensitive to chemotherapy[16] and/or radiation, so surgery remains the treatment of choice if patients are diagnosed at an early stage.[17] If patients have small, but inoperable tumors, they may undergo highly targeted, high intensity radiation therapy. New methods of giving radiation treatment allow doctors to be more accurate in treating lung cancers. This means less radiation affects nearby healthy tissues. New methods include Cyberknife and stereotactic body radiation therapy(SBRT). Certain patients deemed to be higher risk may also receive adjuvant (ancillary) chemotherapy after initial surgery or radiation therapy. There are a number of possible chemotherapy agents which can be selected however most will involve the platinum-based chemotherapy drug called cisplatin.
Other treatments include percutaneous ablation and chemoembolization.[18] The most widely used ablation techniques for lung cancer are radiofrequency ablation, cryoablation, and microwave ablation.[19] Ablation may be an option for patients whose tumors are near the outer edge of the lungs. Nodules less than 1 cm from the trachea, main bronchi, oesophagus and central vessels should be excluded from RFA given high risk of complications and frequent incomplete ablation. Additionally, lesions greater than 5 cm should be excluded and lesions 3 to 5 cm should be considered with caution given high risk of recurrence.[20] As a minimally invasive procedure, it can be a safer alternative for patients who are poor candidates for surgery due to co-morbidities or limited lung function. A study comparing thermal ablation to sublobar resection as treatment for early stage NSCLC in older patients found no difference in overall survival of the patients.[21] It is possible that RFA followed by radiation therapy has a survival benefit due to synergysm of the two mechanisms of cell destruction.[22]
Advanced/metastatic NSCLC
A wide variety of chemotherapies options exist for used in advanced (metastatic) NSCLC.[23] These agents include both traditional chemotherapies like cisplatin which indiscriminately target all rapidly dividing cells as well as newer targeted agents which are more tailored to specific genetic aberrations found within a patient's tumor. At present there are two genetic markers which are routinely profiled in NSCLC tumors to guide further treatment decision making: mutations within EGFR and Anaplastic Lymphoma Kinase.[24] There are also a number of additional genetic markers which are known to be mutated within NSCLC and may impact treatment in the future, including BRAF (gene), HER2/neu and KRAS.
Roughly 10-35% of NSCLC patients will have drug sensitizing mutations of the EGFR.[24] The distribution of these mutations have been found to be race-dependent, with one study estimating that 10% of Caucasians but 50% of Asians will be found to have such tumor markers.[25] A number of different EGFR mutations have been discovered, however certain aberrations will result in hyperactive forms of the protein. Patients with these mutations are more likely to have adenocarcinoma histology and be non-smokers or light smokers. These patients have been shown to be sensitized to certain medications which block the EGFR protein known as tyrosine kinase inhibitors specifically, erlotinib, gefitinib or afatinib.[26]
Thermal ablations i.e. radiofrequency ablation, cryoablation, microwave ablation are appropriate for palliative treatment of tumor-related symptoms or recurrences within treatment fields. Patients with severe pulmonary fibrosis and severe emphysema with a life expectancy <1 year should be considered poor candidates for this treatment.[27]
ALK gene rearrangements
Up to 7% of NSCLC patients have EML4-ALK translocations or mutations in the ROS1 gene; these patients may benefit from ALK inhibitors which are now approved for this subset of patients.[28] Crizotinib gained FDA approval in August 2011 and is an inhibitor of several kinases, specifically ALK, ROS1 and MET. Crizotinib has been shown in clinical studies to have response rates of ~60% if patients are shown to have ALK positive disease.[17] Several studies have also shown that ALK mutations and EGFR activating mutations are typically mutually exclusive. Thus, it is not recommended for patients who fail crizotinib to be switched to an EGFR-targeted drug such as erlotinib.[17]
Other treatment options
NSCLC patients with advanced disease who are not found to have either EGFR or AKT mutations may receive bevacizumab which is a monoclonal antibody medication targeted against the vascular endothelial growth factor (VEGF). This is based on an Eastern Cooperative Oncology Group study which found that adding bevacizumab to carboplatin and paclitaxel chemotherapy for certain patients with recurrent or advanced non-small-cell lung cancer (stage IIIB or IV) may increase both overall survival and progression free survival.[29] The FDA also recently approved the anti-PD-1 agent nivolumab for advanced or metastatic squamous cell carcinoma.[30]
Cause
Smoking is by far the leading risk factor for lung cancer.[31] Cigarette smoke contains more than 6,000 components, many of which lead to DNA damage[32] (see table of tobacco-related DNA damages in Tobacco smoking).
Other causes include radon, non-industrial air-pollution, industrial exposure to some chemicals, and asbestos.[31]
In general, DNA damage appears to be the primary underlying cause of cancer.[33] Though most DNA damages are repairable,[32] leftover un-repaired DNA damages from cigarette smoke are the likely cause of NSCLC.
DNA replication past an un-repaired damage can give rise to a mutation because of inaccurate translesion synthesis. In addition, during repair of DNA double-strand breaks, or repair of other DNA damages, incompletely cleared sites of repair can lead to epigenetic gene silencing.[34][35]
DNA repair deficiency in NSCLC
Deficiencies in DNA repair underlie many forms of cancer.[36] If DNA repair is deficient, the frequency of un-repaired DNA damages will increase and these will tend to cause inaccurate translesion synthesis leading to mutation. Furthermore, increased damages can elevate incomplete repair, leading to epigenetic alterations.
As indicated as in the article Carcinogenesis, mutations in DNA repair genes occasionally occur in cancer. However, deficiencies of DNA repair due to epigenetic alterations that reduce or silence DNA repair gene expression occur much more frequently in cancer.
Epigenetic gene silencing of DNA repair genes occurs frequently in NSCLC. At least nine DNA repair genes that normally function in relatively accurate DNA repair pathways are often repressed by promoter hypermethylation in NSCLC (see table). One DNA repair gene, FEN1, that functions in an inaccurate DNA repair pathway, is expressed at an increased level due to hypo-, rather than hyper-, methylation of its promoter region (deficiency of promoter methylation) in NSCLC (see table).
| Gene | Frequency of hyper- (or hypo-) methylation | DNA repair pathway | Ref. |
|---|---|---|---|
| NEIL1 | 42% | base excision repair | [37] |
| WRN | 38% | homologous recombinational repair, non-homologous end joining, base excision repair | [38] |
| MGMT | 13% - 64% | direct reversal | [37][39][40] |
| ATM | 47% | homologous recombinational repair | [41] |
| MLH1 | 48% - 73% | mismatch repair | [41][42] |
| MSH2 | 42% - 63% | mismatch repair | [41][42] |
| BRCA2 | 42% | homologous recombinational repair | [43] |
| BRCA1 | 30% | homologous recombinational repair | [43] |
| XRCC5 (Ku80) | 20% | non-homologous end joining | [43] |
| FEN1 | 100% hypomethylated (increased expression) | microhomology-mediated end joining | [44] |
The frequent deficiencies in accurate DNA repair, and the increase in inaccurate repair, likely cause the high level of mutation in lung cancer cells of more than 100,000 mutations per genome (see Whole genome sequencing).
References
- ↑ Non-Small Cell Lung Cancer at eMedicine
- ↑ http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-what-is-non-small-cell-lung-cancer
- ↑ Smokers defined as current or former smoker of more than 1 year of duration. See image page in Commons for percentages in numbers. Reference: Table 2 in: Kenfield, S A; Wei, E K; Stampfer, M J; Rosner, B A; Colditz, G A (2008). "Comparison of aspects of smoking among the four histological types of lung cancer". Tobacco Control. 17 (3): 198–204. doi:10.1136/tc.2007.022582. PMC 3044470
 . PMID 18390646.
. PMID 18390646. - 1 2 "Non-small cell lung cancer treatment - National Cancer Institute". Retrieved 2008-10-19.
- ↑ Hanna, Nasser (2007). "Lung Cancer in the Never Smoker Population". Hematology-Oncology. Medscape.
- ↑ Travis, William D; Brambilla, Elisabeth; Muller-Hermelink, H Konrad; et al., eds. (2004). Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart (PDF). World Health Organization Classification of Tumours. Lyon: IARC Press. ISBN 92-832-2418-3. Retrieved 27 March 2010.
- ↑ Simon GR, Turrisi A (September 2007). "Management of small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition)". Chest. 132 (3 Suppl): 324S–339S. doi:10.1378/chest.07-1385. PMID 17873178.
- ↑ Subramanian, J.; Govindan, R. (2007). "Lung Cancer in Never Smokers: A Review". Journal of Clinical Oncology. 25 (5): 561–70. doi:10.1200/JCO.2006.06.8015. PMID 17290066.
- ↑ Mitchell, Richard Sheppard; Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson. "morphology of adenocarcinoma". Robbins Basic Pathology (8th ed.). Philadelphia: Saunders. ISBN 1-4160-2973-7.
- ↑ Travis, William D.; Travis, Lois B.; Devesa, Susan S. (1995). "Lung cancer". Cancer. 75 (1 Suppl): 191–202. doi:10.1002/1097-0142(19950101)75:1+<191::AID-CNCR2820751307>3.0.CO;2-Y. PMID 8000996.
- 1 2 Kenfield, S. A.; Wei, E. K.; Stampfer, M. J.; Rosner, B. A.; Colditz, G. A. (2008). "Comparison of aspects of smoking among the four histological types of lung cancer". Tobacco Control. 17 (3): 198–204. doi:10.1136/tc.2007.022582. PMC 3044470
 . PMID 18390646.
. PMID 18390646. - ↑ Popper, H. H. (2011). "Large cell carcinoma of the lung – a vanishing entity?". Memo - Magazine of European Medical Oncology. 4: 4–9. doi:10.1007/s12254-011-0245-8.
- 1 2 "Cancer Staging Posters: Lung" (PDF). AJCC Cancer Staging (PDF) (7th ed.).
- ↑ De la Guerra, A. "New TNM Classification for Lung Cancer. Part II: A review". Doctors Lounge Website.
- ↑ "National Cancer Institute Non-Small Cell Lung Cancer Treatment (PDQ®)". Retrieved 12 May 2015.
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease. St. Louis MO: Elsevier Saunders. p. 759. ISBN 0-7216-0187-1.
- 1 2 3 "NCCN Clinical Practice Guidelines for NSCLC" (PDF). Retrieved 12 May 2015.
- ↑ "Chemoembolisation". Cancer Research UK.
- ↑ Dupuy , Shulman. "Current Status of Thermal Ablation Treatments for Lung Malignancies". Seminars in Interventional Radiology. 27: 268–275. doi:10.1055/s-0030-1261785.
- ↑ Bargellini; et al. (2011). "Radiofrequency ablation of lung tumours".
- ↑ Kwan; et al. "Thermal Ablation Matches Sublobar Resection Outcomes in Older Patients with Early-stage Non–small Cell Lung Cancer". Journal of Vascular and Interventional Radiology. 25: 1–9.e1. doi:10.1016/j.jvir.2013.10.018.
- ↑ Grieco; et al. "Percutaneous Image-guided Thermal Ablation and Radiation Therapy: Outcomes of Combined Treatment for 41 Patients with Inoperable Stage I/II Non–Small-Cell Lung Cancer". Journal of Vascular and Interventional Radiology. 17: 1117–1124. doi:10.1097/01.RVI.0000228373.58498.6E.
- ↑ Lung Cancer online
- 1 2 "Molecular Profiling of Lung Cancer". Retrieved 12 May 2015.
- ↑ Hirsch, FR; Bunn, PA (May 2009). "EGFR testing in lung cancer is ready for prime time.". The Lancet. Oncology. 10 (5): 432–3. doi:10.1016/s1470-2045(09)70110-x. PMID 19410185.
- ↑ Kris MG (October 2005). "How today's developments in the treatment of non-small cell lung cancer will change tomorrow's standards of care". Oncologist. 10 (Suppl 2): 23–9. doi:10.1634/theoncologist.10-90002-23. PMID 16272456.
- ↑ Dupuy and Shulman. Current Status of Thermal Ablation Treatments for Lung Malignancies. doi: 10.1055/s-0030-1261785
- ↑ Farmer (2010). "Non-Small-Cell Lung Cancer Standards of Care Challenged by a Cornucopia of New Drugs".
- ↑ Sandler, A; Gray, R; Perry, MC; Brahmer, J; Schiller, JH; Dowlati, A; Lilenbaum, R; Johnson, DH (14 December 2006). "Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer.". The New England Journal of Medicine. 355 (24): 2542–50. doi:10.1056/nejmoa061884. PMID 17167137.
- ↑ "FDA Approves Nivolumab for Lung Cancer". Retrieved 12 May 2015.
- 1 2 http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-risk-factors
- 1 2 Liu X, Conner H, Kobayashi T, Kim H, Wen F, Abe S, Fang Q, Wang X, Hashimoto M, Bitterman P, Rennard SI (2005). "Cigarette smoke extract induces DNA damage but not apoptosis in human bronchial epithelial cells". Am. J. Respir. Cell Mol. Biol. 33 (2): 121–9. doi:10.1165/rcmb.2003-0341OC. PMID 15845867.
- ↑ Kastan MB (2008). "DNA damage responses: mechanisms and roles in human disease: 2007 G.H.A. Clowes Memorial Award Lecture". Mol. Cancer Res. 6 (4): 517–24. doi:10.1158/1541-7786.MCR-08-0020. PMID 18403632.
- ↑ O'Hagan, Heather M.; Mohammad, Helai P.; Baylin, Stephen B. (2008). Lee, Jeannie T, ed. "Double Strand Breaks Can Initiate Gene Silencing and SIRT1-Dependent Onset of DNA Methylation in an Exogenous Promoter CpG Island". PLoS Genetics. 4 (8): e1000155. doi:10.1371/journal.pgen.1000155. PMC 2491723
 . PMID 18704159.
. PMID 18704159. - ↑ Cuozzo, Concetta; Porcellini, Antonio; Angrisano, Tiziana; Morano, Annalisa; Lee, Bongyong; Di Pardo, Alba Di; Messina, Samantha; Iuliano, Rodolfo; Fusco, Alfredo; Santillo, Maria R.; Muller, Mark T.; Chiariotti, Lorenzo; Gottesman, Max E.; Avvedimento, Enrico V. (2007). "DNA Damage, Homology-Directed Repair, and DNA Methylation". PLoS Genetics. 3 (7): e110. doi:10.1371/journal.pgen.0030110. PMC 1913100
 . PMID 17616978.
. PMID 17616978. - ↑ Harper JW, Elledge SJ (2007). "The DNA damage response: ten years after". Mol. Cell. 28 (5): 739–45. doi:10.1016/j.molcel.2007.11.015. PMID 18082599.
- 1 2 Do H, Wong NC, Murone C, John T, Solomon B, Mitchell PL, Dobrovic A (2014). "A critical re-assessment of DNA repair gene promoter methylation in non-small cell lung carcinoma". Sci Rep. 4: 4186. doi:10.1038/srep04186. PMC 3935198
 . PMID 24569633.
. PMID 24569633. - ↑ Agrelo R, Cheng WH, Setien F, Ropero S, Espada J, Fraga MF, Herranz M, Paz MF, Sanchez-Cespedes M, Artiga MJ, Guerrero D, Castells A, von Kobbe C, Bohr VA, Esteller M (2006). "Epigenetic inactivation of the premature aging Werner syndrome gene in human cancer". Proc. Natl. Acad. Sci. U.S.A. 103 (23): 8822–7. doi:10.1073/pnas.0600645103. PMC 1466544
 . PMID 16723399.
. PMID 16723399. - ↑ Wolf P, Hu YC, Doffek K, Sidransky D, Ahrendt SA (2001). "O(6)-Methylguanine-DNA methyltransferase promoter hypermethylation shifts the p53 mutational spectrum in non-small cell lung cancer". Cancer Res. 61 (22): 8113–7. PMID 11719438.
- ↑ Ekim M, Caner V, Büyükpınarbaşılı N, Tepeli E, Elmas L, Bağcı G (2011). "Determination of O⁶-methylguanine DNA methyltransferase promoter methylation in non-small cell lung cancer". Genet Test Mol Biomarkers. 15 (5): 357–60. doi:10.1089/gtmb.2010.0211. PMID 21288129.
- 1 2 3 Safar AM, Spencer H, Su X, Coffey M, Cooney CA, Ratnasinghe LD, Hutchins LF, Fan CY (2005). "Methylation profiling of archived non-small cell lung cancer: a promising prognostic system". Clin. Cancer Res. 11 (12): 4400–5. doi:10.1158/1078-0432.CCR-04-2378. PMID 15958624.
- 1 2 Gomes A, Reis-Silva M, Alarcão A, Couceiro P, Sousa V, Carvalho L (2014). "Promoter hypermethylation of DNA repair genes MLH1 and MSH2 in adenocarcinomas and squamous cell carcinomas of the lung". Rev Port Pneumol. 20 (1): 20–30. doi:10.1016/j.rppneu.2013.07.003. PMID 24360395.
- 1 2 3 Lee MN, Tseng RC, Hsu HS, Chen JY, Tzao C, Ho WL, Wang YC (2007). "Epigenetic inactivation of the chromosomal stability control genes BRCA1, BRCA2, and XRCC5 in non-small cell lung cancer". Clin. Cancer Res. 13 (3): 832–8. doi:10.1158/1078-0432.CCR-05-2694. PMID 17289874.
- ↑ Nikolova T, Christmann M, Kaina B (2009). "FEN1 is overexpressed in testis, lung and brain tumors". Anticancer Res. 29 (7): 2453–9. PMID 19596913.