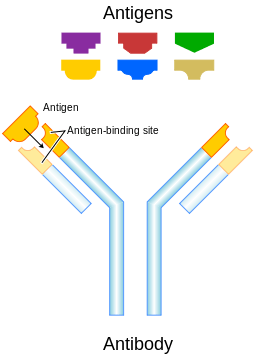
Neutralizing antibody
|
Standard antibody representation. | |
| Properties | |
|---|---|
| Protein Type | Immunoglobin |
| Function | Neutralization of Antigens |
| Production | White B-cells[1][2] |
A neutralizing antibody (NAb) is an antibody that defends a cell from an antigen or infectious body by neutralizing any effect it has biologically.[3] An example of a neutralizing antibody is diphtheria antitoxin, which can neutralize the biological effects of diphtheria toxin.[4]
Neutralization method
Most antibodies work by binding to an antigen, signaling to a white blood cell that this antigen has been targeted, after which the antigen is processed and consequently destroyed. The difference between neutralizing antibodies and binding antibodies is that neutralizing antibodies neutralize the biological effects of the antigen, while binding antibodies flag antigens.[5] This difference can be shown with IFN-beta;
"Antibodies can simply bind to IFN-beta or glatiramer acetate (binding Ab, or BAb) with no subsequent effect on function, or they can block or neutralize (neutralizing Ab, or NAb) their biological activity." --Mark S. Freedman, MD, MSc
This difference is what gives neutralizing antibodies the ability to fight viruses which attack the immune system, since they can neutralize function without a need for white blood cells (excluding production)[5]
Broadly-neutralizing antibodies
Broadly-neutralizing antibodies (bNAbs) affect multiple strains of a particular virus. BNAbs are known for HIV and influenza. Los Alamos National Laboratory's HIV Databases is a comprehensive resource that has a wealth of information about HIV sequences, bNAbs, and more.[6]

Most mutations that shape bNAbs take place at the tips of the Y-shaped antibody molecules, which have loops to ensnare viral epitopes. bNAbs are stickier than other antibodies.
HIV viruses have only about 10 trimers on the surface versus some 450 for influenza. However, bNAbs can compensate by latching on to lipids that make up the viral membrane or even to sugars. bNAb loops are typically longer than ordinary antibodies, increasing the variety of epitopes they can capture. They also accumulate many mutations in the framework region that increase breadth and potency. These mutations do not compromise the antibody's stability, for unknown reasons.[7]
Most bNAb sites are on HIV’s only exposed surface antigen, the flower-like envelope (Env) protein (gp120 and gp41) that sprouts from the viral membrane and is designed to grab and penetrate host cells.
The search for bNAbs has expanded to other diseases, including hepatitis C, dengue and West Nile virus.[7]
History
In 1990, researchers identified the first HIV bNAb, far more powerful that any antibody seen before. They described the exact viral component, or epitope that triggered the antibody. Six amino acids at the tip of HIV's surface protein, gp120, were responsible. The first bNAb turned out to be clinically irrelevant, but in 1994 another team isolated a bNAb that worked on cells taken from patients. This antibody attached to a "conserved" portion of gp120 that outlasts many of its mutations, affecting 17/24 tested strains at low doses. Another bNAb was discovered that acted on protein gp41 across many strains. Antibodies require antigens to trigger them and these were not originally identified.[7]
Over time more bNAbs were isolated, while single cell antibody cloning made it possible to produce large quantities of the antibodies for study. Low levels of bNAbs are now found in up to 25% of HIV patients. bNAbs evolve over years, accumulating some three times as many mutations as other antibodies.[7]
By 2006, researchers had identified a few so-called "broadly neutralizing antibodies" (bNAbs) that worked on multiple HIV strains. They analyzed 1800 blood samples from HIV-infected people from Africa, South Asia and the Anglosphere. They individually probed 30,000 of one woman's antibody-producing B cells and isolated two that were able to stop more than 70% of 162 divergent HIV strains from establishing an infection. Since 2009, researchers have identified more than 50 HIV bNAbs.[7] Integrated web resource BNAber, focused on broadly neutralizing HIV-1 antibodies, has recently been introduced.[8]
In 2006, a Malawi man joined a study within weeks of becoming infected. He repeatedly donated blood over the course of a year, which researches used to create a timeline of changes in his virus' gp120, his antibody response and the ultimate emergence of a bNAb. Researchers want to direct this evolution in other subjects to achieve similar results. A screen of massive gp120 libraries led to one that strongly bound both an original antibody and the mature bNAb that evolved from it. Giving patients a modified gp120 that contains little more than the epitope that both antibodies target could act to "prime" the immune system, followed by a booster that contains trimer spikes in the most natural configuration possible. However, bNAbs have not been shown to prevent an infection.[7]
In 2009, researchers isolated and characterized the first HIV bnabs seen in a decade. The two broadest neutralizers were PGT151 and PGT152. They could block about two-thirds of a large panel of HIV strains. These antibodies did not bind to known epitopes, on Env, gp120 or gp41 (Env's protein subunits) unlike most other bNAbs. Instead they attach to parts of both. Gp120 and gp41 assemble as a trimer. The bNAbs binding site occurs only on the trimer structure, the form of Evn that invades host cells.[9]
Modern testing
Neutralizing antibodies have shown potential in the treatment of retroviral infections. Medical professionals and researchers have shown how the encoding of genes which influence the production of this particular type of antibody could help in the treatment of infections which attack the immune system. Professionals in the field have used HIV treatment as an example of infections these antibodies can treat.[10] Recently, potent and broadly neutralizing human antibodies against influenza (such as CR6261), HIV and hepatitis C have been reported, and have suggested possible strategies to generate an improved vaccine that would confer long-lasting immunity. Another disease which has been linked to the production of neutralizing antibodies is multiple sclerosis.[2] The use of medicines which modify diseases is nothing new, used in regulation for multiple sclerosis since 1998 when the National Multiple Sclerosis Society Consensus Statement recommended their use.[2]
| Disease | Promise | Used? |
|---|---|---|
| Multiple Sclerosis | There is some promise but there have been issues with pharmaceuticals | Yes |
| HIV | Research has shown that NAb's may be able to block viral receptors, for example 2F5 antibody has been shown to block HIV receptors in vitro | No |
Pharmaceutical problems
Although this type of antibody has the ability to fight retroviral infections, in some cases it attacks pharmaceuticals administered to the body which would otherwise treat multiple sclerosis. Recombinant protein drugs, especially those derived from animals, are commonly targeted by neutralizing antibodies. A few examples are Rebif, Betaseron and Avonex.[2]
See also
References
- ↑ Mike Recher; Karl S Lang; Lukas Hunziker; Stefan Freigang; Bruno Eschli; Nicola L Harris; Alexander Navarini; Beatrice M Senn; Katja Fink; Marius Lötscher; Lars Hangartner; Raphaël Zellweger; Martin Hersberger; Alexandre Theocharides; Hans Hengartner; Rolf M Zinkernagel (8 August 2004). "Deliberate removal of T cell help improves virus-neutralizing antibody production". Nature.com. Retrieved 2009-07-30.
- 1 2 3 4 Stachowiak, Julie (August 15, 2008). "Neutralizing Antibodies and Disease-Modifying Therapies for Multiple Sclerosis". About.com. Retrieved 13 June 2009.
- ↑ "Neutralising antibody". Biology-Online. 2008. Retrieved 2009-07-04.
- ↑ "AssessScience". McGraw-Hill. Retrieved 2009-07-04.
- 1 2 Freedman, Mark S. (August 30, 2003). "The Role of Neutralizing Antibodies in MS Treatments". Medscape. Retrieved 2009-08-04.
- ↑ "HIV Databases - lanl.gov".
- 1 2 3 4 5 6 Cohen, J. (2013). "Bound for Glory". Science. 341 (6151): 1168–1171. doi:10.1126/science.341.6151.1168. PMID 24030996.
- ↑ "bnaber.org".
- ↑ "Scientists find new point of attack on HIV for vaccine development". Research & Development. 2014-04-24. Retrieved 2016-06-11.
- ↑ Satiago, Mario L.; Mauricio Montano, Robert Benitez, Ronald J. Messer, at al. (2008). "Apobec3 Encodes Rfv3, a Gene Influencing Neutralizing Antibody Control of Retrovirus Infection". Science Magazine. AAAS. Retrieved 2009-07-04.