Mössbauer spectroscopy

Mössbauer spectroscopy is a spectroscopic technique based on the Mössbauer effect. This effect, discovered by Rudolf Mössbauer in 1958, consists in the nearly recoil-free, resonant absorption and emission of gamma rays in solids.
Like NMR spectroscopy, Mössbauer spectroscopy probes tiny changes in the energy levels of an atomic nucleus in response to its environment. Typically, three types of nuclear interactions may be observed: an isomeric shift, also known as a chemical shift; quadrupole splitting; and magnetic or hyperfine splitting, also known as the Zeeman effect. Due to the high energy and extremely narrow line widths of gamma rays, Mössbauer spectroscopy is a very sensitive technique in terms of energy (and hence frequency) resolution, capable of detecting change in just a few parts per 1011.
Basic principle
Just as a gun recoils when a bullet is fired, conservation of momentum requires a nucleus (such as in a gas) to recoil during emission or absorption of a gamma ray. If a nucleus at rest emits a gamma ray, the energy of the gamma ray is slightly less than the natural energy of the transition, but in order for a nucleus at rest to absorb a gamma ray, the gamma ray's energy must be slightly greater than the natural energy, because in both cases energy is lost to recoil. This means that nuclear resonance (emission and absorption of the same gamma ray by identical nuclei) is unobservable with free nuclei, because the shift in energy is too great and the emission and absorption spectra have no significant overlap. The recoil fraction of the Mössbauer absorption is analyzed by Nuclear resonance vibrational spectroscopy.
Nuclei in a solid crystal, however, are not free to recoil because they are bound in place in the crystal lattice. When a nucleus in a solid emits or absorbs a gamma ray, some energy can still be lost as recoil energy, but in this case it always occurs in discrete packets called phonons (quantized vibrations of the crystal lattice). Any whole number of phonons can be emitted, including zero, which is known as a "recoil-free" event. In this case conservation of momentum is satisfied by the momentum of the crystal as a whole, so practically no energy is lost.[1]
Mössbauer found that a significant fraction of emission and absorption events will be recoil-free, which is quantified using the Lamb–Mössbauer factor.[2] This fact is what makes Mössbauer spectroscopy possible, because it means gamma rays emitted by one nucleus can be resonantly absorbed by a sample containing nuclei of the same isotope, and this absorption can be measured.
Typical method
In its most common form, Mössbauer absorption spectroscopy, a solid sample is exposed to a beam of gamma radiation, and a detector measures the intensity of the beam transmitted through the sample. The atoms in the source emitting the gamma rays must be of the same isotope as the atoms in the sample absorbing them.
If the emitting and absorbing nuclei were in identical chemical environments, the nuclear transition energies would be exactly equal and resonant absorption would be observed with both materials at rest. The difference in chemical environments, however, causes the nuclear energy levels to shift in a few different ways, as described below. Although these energy shifts are tiny (often less than a micro-electronvolt), the extremely narrow spectral linewidths of gamma rays for some radionuclides make the small energy shifts correspond to large changes in absorbance. To bring the two nuclei back into resonance it is necessary to change the energy of the gamma ray slightly, and in practice this is always done using the Doppler effect.
During Mössbauer absorption spectroscopy, the source is accelerated through a range of velocities using a linear motor to produce a Doppler effect and scan the gamma ray energy through a given range. A typical range of velocities for 57Fe, for example, may be ±11 mm/s (1 mm/s = 48.075 neV).[2][3]
In the resulting spectra, gamma ray intensity is plotted as a function of the source velocity. At velocities corresponding to the resonant energy levels of the sample, a fraction of the gamma rays are absorbed, resulting in a drop in the measured intensity and a corresponding dip in the spectrum. The number, positions, and intensities of the dips (also called peaks; dips in transmitted intensity are peaks in absorbance) provide information about the chemical environment of the absorbing nuclei and can be used to characterize the sample.
Selecting a suitable source
Suitable gamma-ray sources consist of a radioactive parent that decays to the desired isotope. For example, the source for 57Fe consists of 57Co, which decays by electron capture to an excited state of 57Fe, which in turn decays to a ground state emitting a gamma-ray of the appropriate energy. The radioactive cobalt is prepared on a foil, often of rhodium.[4] Ideally the parent isotope will have a convenient half-life. Also, the gamma-ray energy should be relatively low, otherwise the system will have a low recoil-free fraction resulting in a poor signal-to-noise ratio and requiring long collection times. The periodic table below indicates those elements having an isotope suitable for Mössbauer spectroscopy. Of these, 57Fe is by far the most common element studied using the technique, although 129I, 119Sn, and 121Sb are also frequently studied.
| H | He | |||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | |||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |||||||||||||||||
| Cs | Ba | La | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | |||||||||||||||||
| Fr | Ra | Ac | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||||||||||||||||
| Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||||||||||||||||||||
| Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
Analysis of Mössbauer spectra
As described above, Mössbauer spectroscopy has an extremely fine energy resolution and can detect even subtle changes in the nuclear environment of the relevant atoms. Typically, there are three types of nuclear interactions that are observed, isomer shift (or chemical shift), quadrupole splitting and hyperfine splitting (or Zeeman splitting).[5][6]
Isomer shift (δ) is a relative measure describing a shift in the resonance energy of a nucleus due to the transition of electrons within its s orbital. The whole spectrum is shifted in either a positive or negative direction depending upon the s electron charge density. This change arises due to alterations in the electrostatic response between the non-zero probability s orbital electrons and the non-zero volume nucleus they orbit.
Only electrons in s orbitals demonstrate non-zero probability because their 3D spherical shape incorporates the volume taken up by the nucleus. However, the p, d, and other electrons may influence the s electron density through a screening effect. The s electron density can also be affected by the oxidation state and the chemical environment of the atom.
Isomer shift (chemical shift, CS) can be expressed using the formula below, where K is a nuclear constant, the difference between Re2 and Rg2 is the effective nuclear charge radius difference between excited state and the ground state, and the difference between [Ψs2(0)]a and [Ψs2(0)]b is the electron density difference on the nucleus (a = source, b = sample). Isomer shift does not change with temperature, however, small changes are generally attributed to second-order Doppler effect.

The physical meaning of this equation can be clarified using examples:
- While an increase in s electron density in 57Fe spectrum gives a negative shift because the change in the effective nuclear charge is negative (owing to Re < Rg), an increase in s electron density in 119Sn gives a positive shift due to a positive change in overall nuclear charge (owing to Re > Rg).
- Oxidised ferric ions (Fe3+) have lower isomer shifts than ferrous ions (Fe2+) because s electron density at the nucleus of ferric ions is greater due to a weaker screening effect by d electrons.[7]
The isomer shift is useful for determining oxidation state, valency states, electron shielding and the electron-drawing power of electronegative groups.[5]
Quadrupole splitting reflects the interaction between the nuclear energy levels and surrounding electric field gradient (EFG). Nuclei in states with non-spherical charge distributions, i.e. all those with angular quantum number (I) greater than 1/2, produce an asymmetrical electric field (produced by an asymmetric electronic charge distribution or ligand arrangement) which splits the nuclear energy levels. This produces a nuclear quadrupole moment.[5]
In the case of an isotope with a I=3/2 excited state, such as 57Fe or 119Sn, the 3/2 to 1/2 transition is split into two substates mI=±1/2 and mI=±3/2. These appear as two specific peaks in a spectrum, sometimes referred to as a 'doublet'. Quadrupole splitting is measured as the separation between these two peaks and reflects the character of the electric field at the nucleus. Despite quadrupole splitting might be difficult to calculate, there are algorithms to calculate it numerically.[8]
The Quadrupole splitting can be used for determining oxidation state, spin state, site symmetry and the arrangement of ligands.[5]

Magnetic splitting (hyperfine splitting) is a result of the interaction between the nucleus and any surrounding magnetic field. A nucleus with spin, I, splits into 2I + 1 sub-energy levels in the presence of magnetic field. For example, a nucleus with spin state I= 3/2 will split into 4 non-degenerate sub-states with mI values of +3/2, +1/2, -1/2 and −3/2. Each split is hyperfine, being in the order of 10−7eV. The selection rule of magnetic dipoles means that transitions between the excited state and ground state can only occur where mI changes by 0 or 1. This gives six possible transitions for a 3/2 to 1/2 transition.[5] Generally speaking therefore, in the majority of cases only six peaks can be monitored in a spectrum produced by a hyperfine splitting nucleus.
The three Mössbauer parameters: isomer shift, quadrupole splitting, and hyperfine splitting can often be used to identify a particular compound by comparson to spectra for standards. A large database including most of the published Mössbauer parameters is maintained by the Mössbauer Effect Data Center.[9] In some cases, a compound may have more than one possible position for the Mössbauer active atom. For example, the crystal structure of magnetite (Fe3O4) supports two different sites for the iron atoms. The corresponding spectrum therefore has twelve peaks, a 'hextet' for each potential atomic site. Thus, magnetite also has two sets of Mössbauer parameters, one for each site.
Combination of all: Many times all effects are observed: isomer shift, quadrupole splitting, and magnetic Zeeman effect. In such cases the isomer shift is given by the average of all lines. The quadrupole splitting when all the four excited substates are equally shifted (two substates are lifted and other two are lowered) is given the shift of the outer two lines relative to the inner two lines (generally innermost two lines are not considered). If the shifting of four substates are not equal then the quadrupole splitting is often extracted using fitting software where all the six lines are taken into account.
In addition, the relative intensities of the various peaks reflect the relative concentrations of compounds in a sample and can be used for semi-quantitative analysis. Also, since ferromagnetic phenomena are size-dependent, in some cases spectra can provide insight into the crystallite size and grain structure of a material.
Mössbauer emission spectroscopy
Mossbauer emission spectroscopy is a specialized variant of Mossbauer spectroscopy where the emitting element is in the one being probed, and the absorbing element is the reference. Most commonly, the technique is applied to the 57Co/57Fe pair. A typical application is the characterization of the cobalt sites in amorphous Co-Mo catalysts used in hydrodesulfurization. In such a case, the sample is doped with 57Co.[10]
Applications of Mössbauer spectroscopy
Among the drawbacks of the technique are the limited number of gamma ray sources and the requirement that samples be solid in order to eliminate the recoil of the nucleus. Mössbauer spectroscopy is unique in its sensitivity to subtle changes in the chemical environment of the nucleus including oxidation state changes, the effect of different ligands on a particular atom, and the magnetic environment of the sample.
As an analytical tool Mössbauer spectroscopy has been especially useful in the field of geology for identifying the composition of iron-containing specimens including meteors and moon rocks. In situ data collection of Mössbauer spectra has also been carried out on iron rich rocks on Mars.[11]
In another application, Mössbauer spectroscopy is used to characterize phase transformations in iron catalysts, e.g., those used for Fischer–Tropsch synthesis. While initially consisting of hematite (Fe2O3), these catalysts transform into a mixture of magnetite (Fe3O4) and several iron carbides. The formation of carbides appears to improve catalytic activity, however it can also lead to the mechanical break-up and attrition of the catalyst particles, which can cause difficulties in the final separation of catalyst from reaction products.[12]
Mössbauer spectroscopy has also been used to determine the relative concentration change in the oxidation state of antimony (Sb) during the selective oxidation of olefins. During calcination all the Sb ions in an antimony-containing tin dioxide catalyst transform into the +5 oxidation state. Following the catalytic reaction, almost all Sb ions revert from the +5 to the +3 oxidation state. A significant change in the chemical environment surrounding the antimony nucleus occurs during the oxidation state change which can easily be monitored as an isomer shift in the Mössbauer spectrum.[13]
This technique has also been used to observe the second-order transverse Doppler effect predicted by the theory of relativity, because of very high energy resolution.[14]
Bioinorganic chemistry
Mossbauer spectroscopy has been widely applied to bioinorganic chemistry, especially for the study of iron-containing proteins and enzymes. Often the technique is used to determine the oxidation state of iron. Examples of prominent iron-containing biomolecules are iron-sulfur proteins, ferritin, hemes including the cytochromes. These studies are often supplemented by analysis of related model complexes.[15][16] An area of particular interest is the characterization of intermediates involved in oxygen activation by iron proteins.[17]
Vibrational spectra of iron-57 enriched biomolecules can be acquired using nuclear resonance vibrational spectroscopy(NRVS) in which the sample is scanned through a range of synchrotron-generated X-rays, centered at the Mossbauer absorbance frequency. Stokes and antistokes peaks in the spectrum correspond to low frequency vibrational, many below 600 cm−1 with some below 100 cm−1.
Mössbauer spectrometers

A Mössbauer spectrometer is a device that performs Mössbauer spectroscopy, or a device that uses the Mössbauer effect to determine the chemical environment of Mössbauer nuclei present in the sample. It is formed by three main parts; a source that moves back and forth to generate a doppler effect, a collimator that filters out non-parallel gamma rays and a detector.
A miniature Mössbauer Spectrometer, named (MB) MIMOS II, was used by the two rovers in NASA's Mars Exploration Rover missions.[18]
57Fe Mössbauer spectroscopy
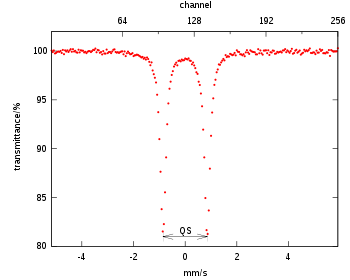
The Mössbauer parameters: chemical isomer shift and quadrupole splitting are generally evaluated with respect to a reference material. For example, in iron compounds, the Mössbauer parameters were evaluated using iron foil (thickness less than 40 micrometers). The centroid of the six lines spectrum from metallic iron foil is −0.1 mm/s (for Co/Rh source). All shifts in other iron compounds are computed relative to this −0.10 mm/s (at room temperature), i.e., in this case isomer shifts are relative to Co/Rh source. In other words, the centre point of the Mössbauer spectrum is zero. The shift values may also be reported relative to 0.0 mm/s, here shifts are relative to the iron foil.
To calculate outer line distance from six line iron spectrum:
where c is the speed of light, Bint is the internal magnetic field of the metallic iron (33 T), μN is the nuclear magneton (3.1524512605×10−8 eV/T), Eγ is the excitation energy (14.412497(3) keV[19])
, gn is the ground state nuclear splitting factor (0.09062/(I), where Isospin I = 1⁄2) and g*
n is the excited state splitting factor of 57Fe (0.1549/(I), where I = 3⁄2).
By substituting the above values one would get V = 10.6258 mm/s.
Other values are sometimes used to reflect different qualities of iron foils. In all cases any change in V only affects the isomer shift and not the quadrupole splitting. As the IBAME, the authority for Mössbauer spectroscopy, does not specify a particular value, anything between 10.60 mm/s to 10.67 mm/s can be used. For this reason it is highly recommended to provide the isomer shift values with respect to the source used, mentioning the details of source (centre of gravity of the folded spectrum), instead with respect to iron foil.
References
- ↑ International Board on the Applications of the Mössbauer Effect (IBAME) and Mössbauer Effect Data Center (MEDC), Mössbauer Effect website Accessed June 3, 2010
- 1 2 Gütlich, J.M.; The Principle of the Mössbauer Effect and Basic Concepts of Mössbauer Spectrometry
- ↑ Mössbauer Spectroscopy Group, Royal Society of Chemistry (RSC) website, Introduction to Mössbauer Spectroscopy Part 1 Accessed June 3, 2010
- ↑ Longworth, G; Window, B (1971). "The preparation of narrow-line Mössbauer sources of 57Co in metallic matrices". Journal of Physics D. 4 (6): 835. Bibcode:1971JPhD....4..835L. doi:10.1088/0022-3727/4/6/316.
- 1 2 3 4 5 Mössbauer Spectroscopy Group, Royal Society of Chemistry (RSC) website, Introduction to Mössbauer Spectroscopy Part 2 Accessed June 3, 2010
- ↑ P. Gütlich, J.M. Greneche, F.J. Berry; Mössbauer Spectroscopy: A Powerful Tool in Scientific Research Accessed June 3, 2010
- ↑ Walker, L.; Wertheim, G.; Jaccarino, V. (1961). "Interpretation of the Fe57 Isomer Shift". Physical Review Letters. 6 (3): 98. Bibcode:1961PhRvL...6...98W. doi:10.1103/PhysRevLett.6.98.
- ↑ Hernandez-Gomez, J J; Marquina, V; Gomez, R W (25 July 2013). "Algorithm to compute the electric field gradient tensor in ionic crystals". Rev. Mex. Fís. Sociedad Mexicana de Física. 58 (1): 13. Retrieved 23 April 2016.
- ↑ Mössbauer Effect Data Center
- ↑ D.L. Nagy "Trends in Moessbauer emission spectroscopy of 57Co/57Fe" Hyperfine Interactions 1994, volume 83, pp. 3-19. doi: 10.1007/BF02074255
- ↑ Klingelhöfer, G. (2004). "Mössbauer in situ studies of the surface of Mars". Hyperfine Interactions. 158 (1–4): 117–124. Bibcode:2004HyInt.158..117K. doi:10.1007/s10751-005-9019-1.
- ↑ Sarkar, A.; et al. (2007). "Fischer–Tropsch Synthesis: Characterization Rb Promoted Iron Catalyst". Catalysis Letters. 121 (1–2): 1–11. doi:10.1007/s10562-007-9288-1.
- ↑ Burger, K.; Nemes-Vetéssy, Zs.; Vértes, A.; Afanasov, M. I. (1986). "Mössbauer spectroscopic study of the oxidation state of antimony in antimony sulfides of different composition". Journal of Crystallographic and Spectroscopic Research. 16 (2): 295. doi:10.1007/BF01161115.
- ↑ Chen, Y.-L.; Yang, D.-P. (2007). "Recoilless Fraction and Second-Order Doppler Effect". Mössbauer Effect in Lattice Dynamics. John Wiley & Sons. doi:10.1002/9783527611423.ch5. ISBN 978-3-527-61142-3.
- ↑ Martinho, M., Munck, E., "57Fe Mossbauer spectroscopy in chemistry and biology," in 2010, John Wiley & Sons, Inc..doi:10.1002/9780470602539.ch2
- ↑ Schuenemann, V., Paulsen, H., "Moessbauer spectroscopy," in 2007, John Wiley & Sons Ltd. ISBN 978-0-470-03217-6.
- ↑ Miquel Costas, Mark P. Mehn, Michael P. Jensen, and Lawrence Que, Jr. "Dioxygen Activation at Mononuclear Nonheme Iron Active Sites: Enzymes, Models, and Intermediates" Chem. Rev., 2004, 104 (2), pp 939–986. doi:10.1021/cr020628n
- ↑ Klingelhöfer, G.; et al. (2002). "The miniaturized Mössbauer spectrometer MIMOS II for extraterrestrial and outdoor terrestrial applications: A status report". Hyperfine Interactions. 144 (1–4): 371–379. Bibcode:2002HyInt.144..371K. doi:10.1023/A:1025444209059.
- ↑ Mössbauer Effect Data Center 20.08.2013
External links
- Mössbauer Effect Data Center page, including periodic table of Mössbauer isotopes
- Introduction to Mössbauer Spectroscopy — RSC site
- Mössbauer Spectroscopy: A Powerful Tool in Scientific Research
- "Mossbauer Spectroscopy--A Rewarding Probe of Morphological Structure of Semiconducting Glasses ", P. Boolchand in Physical Properties of Amorphous Materials (Institute for Amorphous Studies Series), Springer US, Eds.: David Adler, Brian B. Schwartz, Martin C. Steele. [PDF]