Denticity

monodentate ligands
Denticity refers to the number of donor groups in a single ligand that bind to a central atom in a coordination complex.[1][2] In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be monodentate (sometimes called unidentate). Ligands with more than one bonded atom are called polydentate or multidentate. The word denticity is derived from dentis, the Latin word for tooth. The ligand is thought of as biting the metal at one or more linkage points. The denticity of a ligand is described with the Greek letter κ ('kappa').[3] For example, κ6-EDTA describes an EDTA ligand that coordinates through 6 non-contiguous atoms.
Denticity is different from hapticity because hapticity refers exclusively to ligands where the coordinating atoms are contiguous. In these cases the η ('eta') notation is used.[4] Bridging ligands use the μ ('mu') notation.[5][6]
Classes of denticity
Polydentate ligands are chelating agents[7] and classified by their denticity. Some atoms cannot form the maximum possible number of bonds a ligand could make. In that case one or more binding sites of the ligand are unused. Such sites can be used to form a bond with another chemical species.
- Bidentate (also called didentate) ligands bind with two atoms, an example being ethylenediamine.
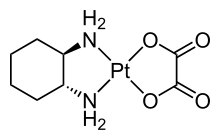 Structure of the pharmaceutical Oxaliplatin, which features two different bidentate ligands.
Structure of the pharmaceutical Oxaliplatin, which features two different bidentate ligands.
- Tridentate ligands bind with three atoms, an example being terpyridine. Tridentate ligands usually bind via two kinds of connectivity, called "mer" and "fac." Cyclic tridentate ligands such as TACN and 9-ane-S3 bind in a facial manner.
- Tetradentate ligands bind with four atoms, an example being triethylenetetramine (abbreviated trien). Tetradentate ligands bind via three connectivities depending on their topology and the geometry of the metal center. For octahedral metals, the linear tetradentate trien can bind via three geometries. Tripodal tetradentate ligands, e.g. tris(2-aminoethyl)amine, are more constrained, and on octahedra leave two cis sites. Many naturally occurring macrocyclic ligands are tetradentative, an example being the porphyrin in heme.
- Pentadentate ligands bind with five atoms, an example being ethylenediaminetriacetic acid.
- Hexadentate ligands bind with six atoms, an example being EDTA (although it can bind in a tetradentate manner).
- Ligands of denticity greater than 6 are well known. The ligands 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate (DOTA) and diethylene triamine pentaacetate (DTPA) are octadentate. They are particularly useful for binding lanthanide ions, which typically have coordination numbers greater than 6.

Stability constants
In general, the stability of a metal complex correlates with the denticity of the ligands. The stability of a complex is represented quantitatively in the form of Stability constants. Hexadentate ligands tend to bind metal ions more strongly than ligands of lower denticity.
See also
External links
- EDTA chelation lecture notes. 2.4MB PDF - Slide 3 on denticity
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "denticity".
- ↑ von Zelewsky, A. "Stereochemistry of Coordination Compounds" John Wiley: Chichester, 1995. ISBN 047195599X.
- ↑ http://goldbook.iupac.org/K03366.html
- ↑ http://goldbook.iupac.org/H01881.html
- ↑ http://goldbook.iupac.org/B00741.html>
- ↑ http://goldbook.iupac.org/M03659.html
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "chelation".