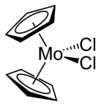
Molybdocene dichloride
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
dichlorobis(η5-cyclopentadienyl)molybdenum(IV) | |||
| Other names
molybdocene dichloride, molybdenocene dichloride, dichloridobis(cyclopentadienyl)molybdenum(IV) | |||
| Identifiers | |||
| 12184-22-4 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 398274 | ||
| ECHA InfoCard | 100.159.644 | ||
| PubChem | 452153 | ||
| |||
| |||
| Properties | |||
| C10H10Cl2Mo | |||
| Molar mass | 297.04 g·mol−1 | ||
| Appearance | greenish-brown powder | ||
| insoluble, moisture sensitive | |||
| Hazards | |||
| Safety data sheet | [1] | ||
| Related compounds | |||
| Related compounds |
Ferrocene Zirconocene dichloride Vanadocene dichloride Niobocene dichloride Titanocene dichloride Tantalocene dichloride | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Molybdocene dichloride is the organomolybdenum compound with the formula (η5-C5H5)2MoCl2 and IUPAC name dichlorobis(η5-cyclopentadienyl)molybdenum(IV), and is commonly abbreviated as Cp2MoCl2. It is a brownish-green air- and moisture-sensitive powder. In the research laboratory, it is used to prepare many derivatives.
Preparation and structure
The compound was first reported in 1964 by Malcolm Green and his student Cooper.[2] It is prepared from molybdocene dihydride, which in turn is prepared by a famously complex reaction involving molybdenum pentachloride, sodium cyclopentadienide, and sodium borohydride. The dihydride converts to the dichloride upon treatment with chloroform:[3]
- (C5H5)2MoH2 + 2 CHCl3 → (C5H5)2MoCl2 + 2 CH2Cl2
The compound adopts a "clamshell" structure where the Cp rings are not parallel, the average Cp(centroid)-M-Cp angle being 130.6°. The two chloride ligands are cis, the Cl-Mo-Cl angle of 82° being narrower than in niobocene dichloride (85.6°), which in turn is less than in zirconacene dichloride (92.1°). This trend helped to establish the orientation of the HOMO in this class of complex.[4]
Uses
Unlike the titanocene and zirconacene derivatives, the molybddocene compounds have yielded no commercial applications.
All metallocene dihalides exhibit some anti-cancer activity,[5] but these have not yielded useful compounds in the clinic.[6]
References
- ↑ "42-0100 Molybdenum " Bis(cyclopentadienyl)molybdenum dichloride, 99%". Strem Chemicals. 27 July 2011. Retrieved 5 February 2012.
- ↑ R. L. Cooper and M. L. H. Green, Z. Naturforsch., B: Anorg. Chem. Org. Chem. 1964, volume 19, pp. 652. R. L. Cooper and M. L. H. Green, J. Chem. Soc. (A), 1967, p. 1155.
- ↑ Silavwe, Ned D.; Castellani, Michael P.; Tyler, David R. (1992). "Bis(η5-Cyclopentadienyl)Molybdenum(IV) Complexes". Inorganic Syntheses. 29: 204–211. doi:10.1002/9780470132609.ch50.
- ↑ K. Prout, T. S. Cameron, R. A. Forder, and in parts S. R. Critchley, B. Denton and G. V. Rees "The crystal and molecular structures of bent bis-π-cyclopentadienyl-metal complexes: (a) bis-π-cyclopentadienyldibromorhenium(V) tetrafluoroborate, (b) bis-π-cyclopentadienyldichloromolybdenum(IV), (c) bis-π-cyclopentadienylhydroxomethylaminomolybdenum(IV) hexafluorophosphate, (d) bis-π-cyclopentadienylethylchloromolybdenum(IV), (e) bis-π-cyclopentadienyldichloroniobium(IV), (f) bis-π-cyclopentadienyldichloromolybdenum(V) tetrafluoroborate, (g) μ-oxo-bis[bis-π-cyclopentadienylchloroniobium(IV)] tetrafluoroborate, (h) bis-π-cyclopentadienyldichlorozirconium" Acta Crystallogr. 1974, volume B30, pp. 2290–2304. doi:10.1107/S0567740874007011
- ↑ Roat-Malone, R. M. (2007). Bioinorganic Chemistry: A Short Course (2nd ed.). John Wiley & Sons. pp. 19–20. ISBN 978-0-471-76113-6.
- ↑ Waern, J. B.; Dillon, C. T.; Harding, M. M. (2005). "Organometallic Anticancer Agents: Cellular Uptake and Cytotoxicity Studies on Thiol Derivatives of the Antitumor Agent Molybdocene Dichloride". J. Med. Chem. 48 (6): 2093–2099. doi:10.1021/jm049585o.
| Wikimedia Commons has media related to Molybdocene dichloride. |