Molecular solid
A molecular solid is a solid composed of molecules held together by the van der Waals forces. Because these dipole forces are weaker than covalent or ionic bonds, molecular solids are soft and have relatively low melting temperature. Molecular solids can be amorphous solids or crystals. Examples of molecular solids include hydrocarbons, ice, sugar, fullerenes, sulfur, and solid carbon dioxide. Pure molecular solids are electrical insulators but they can be made conductive by doping.
Other classes of solid include ionic solids, held together by ionic bonding; glasses, ceramics, and polymeric solids, held together by covalent bonds; and metals, held together by metallic bonding.
Structure and composition
| Formula | Tm °C |
|---|---|
| H2 | −259.1 |
| F2 | −219.6 |
| O2 | −218.8 |
| N2 | −210.0 |
| CH4 | −182.4 |
| C2H6 | −181.8 |
| C3H8 | −165.0 |
| C4H10 | −138.3 |
| C5H12 | −129.8 |
| Cl2 | −101.6 |
| C6H14 | −95.3 |
| HBr | −86.8 |
| HF | −80.0 |
| NH3 | −80.0 |
| HI | −50.8 |
| C10H22 | −29.7 |
| HCl | −27.3 |
| Br2 | −7.2 |
| H2O | 0.0 |
| C6H6 | 5.5 |
| I2 | 113.7 |
| S8 | 119.0 |
| C6Cl6 | 220.0 |
- See also
higher alkanes
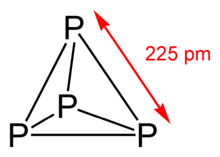
The term "molecular solid" may refer not to a certain chemical composition, but to a specific form of a material. For example, solid phosphorus can crystallize in different allotropes called "white", "red", and "black" phosphorus. White phosphorus forms molecular crystals composed of tetrahedral P4 molecules.[3] Heating at ambient pressure to 250 °C or exposing to sunlight converts white phosphorus to red phosphorus where the P4 tetrahedra are no longer isolated, but are connected by covalent bonds into polymer-like chains.[4] Heating white phosphorus under high (GPa) pressures converts it to black phosphorus which has a layered, graphite-like structure.[5][6]
The structural transitions in phosphorus are reversible: upon releasing high pressure, black phosphorus gradually converts into the red allotrope, and by vaporizing red phosphorus at 490 °C in inert atmosphere and condensing the vapor, covalent red phosphorus can be transformed back into the white molecular solid.[7]
 |
 |
 | |||
| White, red, violet, and black phosphorus samples | Structure unit of white phosphorus |
Structures of red | violet | and black phosphorus |
Similarly, yellow arsenic is a molecular solid composed of As4 units; it is metastable and gradually transforms into gray arsenic upon heating or illumination.[8] Some forms of sulfur and selenium are composed of S8 (or Se8) units and are molecular solids at ambient conditions, but they can convert into covalent allotropes having atomic chains extending all through the crystal.[9][10]
Changes in the chemical composition can have even stronger effects on the bonding in solids. For example, whereas both hydrogen and lithium belong to the first group of the periodic table, LiCl is ionic and HCl is a molecular solid.[11]
Examples of molecular solids
Several classes of molecular solids can be distinguished (see table right). The vast majority of molecular solids can be attributed to organic compounds containing carbon and hydrogen, such as hydrocarbons (CnHm) and diamondoids.[12][13] Spherical molecules consisting of different number of carbon atoms, that is fullerenes, are another important class. Less numerous, yet distinctive molecular solids are halogens (e.g. Cl2) and their compounds with hydrogen (HCl), as well as light chalcogens (O2) and pnictogens (N2).
Properties
Weakness of intermolecular forces results in low melting temperatures of molecular solids. Whereas the characteristic melting point of metals and ionic solids is ~1000 °C, most molecular solids melt well below ~300 °C (see table), thus many corresponding substances are either liquid (ice) or gaseous (oxygen) at room temperature.[14] Molecular solids also have relatively low density and hardness. This is because of the light elements involved and relatively long and thus weak intermolecular bonds. Because of the charge neutrality of the constituent molecules and long distance between them, molecular solids are electrical insulators.[1]
The above tendencies can be illustrated on example of different allotropes of phosphorus. White phosphorus, a molecular solid, has a relatively low density of 1.82 g/cm3 and melting point of 44.1 °C; it is a soft material which can be cut with a knife. When it is converted to the covalent red phosphorus, the density goes to 2.2–2.4 g/cm3 and melting point to 590 °C, and when white phosphorus is transformed into the (also covalent) black phosphorus, the density becomes 2.69–3.8 g/cm3 and melting temperature ~200 °C. Both red and black phosphorus forms are significantly harder than white phosphorus,[15] and whereas white phosphorus is an insulator, the black allotrope, which consists of layers extending over the whole crystal, does conduct electricity.[16][17]
Conductivity of molecular solids can be illustrated on example of fullerene. Its solid is an insulator because all valence electrons of carbon atoms are involved into the covalent bonds within the individual carbon molecules. However, inserting (intercalating) alkali metal atoms between the fullerene molecules provides extra electrons, which can be easily ionized from the metal atoms and make material conductive and even superconductive.[18]
-
Crystal structure of hexagonal ice. Gray dashed lines indicate hydrogen bonds.
-

Unit cell of solid carbon dioxide, a molecular solid containing discrete CO2 molecules
-

Structure of solid iodine
-

Model of sucrose molecule
-
Model of a solid fullerene intercalated with alkali metal atoms (green)
-

Ice
-

Small pellets of dry ice subliming in air
-
Crystalline iodine
-

Sucrose crystals
-
Fullerene crystals
See also
References
- 1 2 James Wei (2007). Product engineering: molecular structure and properties. Oxford University Press. p. 137. ISBN 0-19-515917-9.
- ↑ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ↑ John Olmsted, Gregory M. Williams (1997). Chemistry: the molecular science. Jones & Bartlett Learning. p. 981. ISBN 0-8151-8450-6.
- ↑ Singhal Atul. The Pearson Guide to Objective Chemistry for the AIEEE. p. 36. ISBN 81-317-1359-8.
- ↑ Gary Wulfsberg (1991). Principles of descriptive inorganic chemistry. University Science Books. p. 186. ISBN 0-935702-66-0.
- ↑ Simon, Arndt; Borrmann, Horst; Horakh, Jörg (1997). "On the Polymorphism of White Phosphorus". Chemische Berichte. 130: 1235. doi:10.1002/cber.19971300911.
- ↑ AK Srivastava and PC Jain. Chemistry Vol (1 and 2). FK Publications. p. 548. ISBN 81-88597-83-X.
- ↑ Holleman, Arnold F; Wiberg, Egon; Wiberg, Nils (1985). "Arsen". Lehrbuch der Anorganischen Chemie (in German) (91–100 ed.). Walter de Gruyter. pp. 675–681. ISBN 3-11-007511-3.
- ↑ Masters, Anthony F. "Allotropes – Group 13, Group 14, Group 15, Group 16". Chemistry Explained. Retrieved 2009-01-06.
- ↑ James E. House (2008). Inorganic chemistry. Academic Press. p. 524. ISBN 0-12-356786-6.
- ↑ Steven S. Zumdahl (2007). Chemical principles. Cengage Learning. p. 60. ISBN 0-618-94690-X.
- ↑ Markle, R. C. (2000). "Molecular building blocks and development strategies for molecular nanotechnology". Nanotechnology. 11: 89. doi:10.1088/0957-4484/11/2/309.
- ↑ Garcia, J. C.; Justo, J. F.; Machado, W. V. M.; Assali, L. V. C. (2009). "Functionalized adamantane: building blocks for nanostructure self-assembly". Phys. Rev. B. 80: 125421. doi:10.1103/PhysRevB.80.125421.
- ↑ Darrell D. Ebbing, Steven D. Gammon (2007). General Chemistry. Cengage Learning. p. 446. ISBN 0-618-85748-6.
- ↑ AK Srivastava and PC Jain. Chemistry Vol (1 and 2). FK Publications. p. 550. ISBN 81-88597-83-X.
- ↑ Dale L. Perry, Sidney L. Phillips (1995). Handbook of inorganic compounds. CRC Press. p. 293. ISBN 0-8493-8671-3.
- ↑ Perrin Walker, William H. Tarn (1991). CRC handbook of metal etchants. CRC Press. p. 900. ISBN 0-8493-3623-6.
- ↑ O. Gunnarsson (1997). "Superconductivity in fullerides". Rev. Mod. Phys. 69: 575. arXiv:cond-mat/9611150
 . Bibcode:1997RvMP...69..575G. doi:10.1103/RevModPhys.69.575.
. Bibcode:1997RvMP...69..575G. doi:10.1103/RevModPhys.69.575.