Markovnikov's rule

In organic chemistry, Markovnikov's rule or Markownikoff's rule describes the outcome of some addition reactions. The rule was formulated by Russian chemist Vladimir Vasilevich Markovnikov in 1870.[1][2]
Explanation
The rule states that with the addition of a protic acid HX to an asymmetric alkene, the acid hydrogen (H) becomes attached to the carbon with fewer alkyl substituents, and the halide (X) group becomes attached to the carbon with more alkyl substituents. Alternatively, the rule can be stated that the hydrogen atom is added to the carbon with the greatest number of hydrogen atoms while the X component is added to the carbon with the least number of hydrogen atoms.[3]
The same is true when an alkene reacts with water in an addition reaction to form an alcohol which involve formation of carbocations. The hydroxyl group (OH) bonds to the carbon that has the greater number of carbon–carbon bonds, while the hydrogen bonds to the carbon on the other end of the double bond, that has more carbon–hydrogen bonds.
The chemical basis for Markovnikov's Rule is the formation of the most stable carbocation during the addition process. The addition of the hydrogen ion to one carbon atom in the alkene creates a positive charge on the other carbon, forming a carbocation intermediate. The more substituted the carbocation, the more stable it is, due to induction and hyperconjugation. The major product of the addition reaction will be the one formed from the more stable intermediate. Therefore, the major product of the addition of HX (where X is some atom more electronegative than H) to an alkene has the hydrogen atom in the less substituted position and X in the more substituted position. But the other less substituted, less stable carbocation will still be formed at some concentration, and will proceed to be the minor product with the opposite, conjugate attachment of X.
Anti-Markovnikov reactions
Mechanisms that avoid the carbocation intermediate may react through other mechanisms that are regioselective, not predicted by Markovnikov's rule, such as free radical addition. Such reactions are said to be anti-Markovnikov, since the halogen adds to the less substituted carbon, exactly the opposite of a Markovnikov reaction. Physically, like the positive charge, the radical is most stable when it is in the more substituted position. The anti-Markovnikov rule can be explained best by taking an example of hydrogen bromide addition to propene in the presence of benzoyl peroxide. The reaction of HBr with substituted alkenes was prototypical in the study of free-radical additions. Early chemists discovered that the reason for the variability in the ratio of Markovnikov to anti-Markovnikov reaction products was due to the unexpected presence of free radical ionizing substances such as peroxides. The explanation is that HBr produces a Br radical, which then reacts with the double bond. Since the bromine atom is relatively sizable, it is more likely to encounter and react with the least substituted carbon. In this case the terminal carbon is a reactant which produces a primary addition product instead of a secondary addition product, in the case of propene.
A new method of anti-Markovnikov addition has been described by Hamilton and Nicewicz, who utilize aromatic molecules and light from a low-energy diode to turn the alkene into a cation radical.[4] [5]
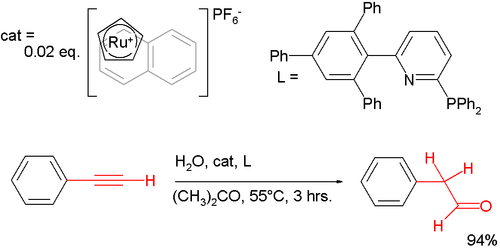
Anti-Markovnikov behaviour extends to more chemical reactions than simply additions to alkenes. One anti-Markovnikov manifestation is observed in the hydration of phenylacetylene by auric catalysis, which gives regular acetophenone; although with a special ruthenium catalyst[6] it provides the other regioisomer 2-phenylacetaldehyde:[7]
Anti-Markovnikov behavior can also manifest itself in certain rearrangement reactions. In a titanium(IV) chloride-catalyzed formal nucleophilic substitution at enantiopure 1 in the scheme below, two products are formed – 2a and 2b. Due to the two chiral centers in the target molecule, the carbon carrying chlorine and the carbon carrying the methyl and acetoxyethyl group, four different compounds are to be formed: 1R,2R- (drawn as 2b) 1R,2S- 1S,2R- (drawn as 2a) and 1S,2S- . Therefore both of the depicted structures will exist in a D- and an L-form. :[8]

This product distribution can be rationalized by assuming that loss of the hydroxy group in 1 gives the tertiary carbocation A, which rearranges to the seemingly less stable secondary carbocation B. Chlorine can approach this center from two faces leading to the observed mixture of isomers.
Another notable example of anti-Markovnikov addition is hydroboration.
References
- ↑ W. Markownikoff (1870). "Ueber die Abhängigkeit der verschiedenen Vertretbarkeit des Radicalwasserstoffs in den isomeren Buttersäuren". Annalen der Pharmacie. 153 (1): 228–59. doi:10.1002/jlac.18701530204.
- ↑ Hughes, Peter (2006). "Was Markovnikov's Rule an Inspired Guess?". Journal of Chemical Education. 83 (8): 1152. Bibcode:2006JChEd..83.1152H. doi:10.1021/ed083p1152.
- ↑ Organic Chemistry, 6th Edition, by John McMurry. Section 6.9, page 187
- ↑ http://cen.acs.org/articles/91/i15/Light-Driven-Reaction-Modifies-Double.html
- ↑ http://pubs.acs.org/doi/abs/10.1021/ja309635w?source=cen
- ↑ catalyst system based on in-situ reaction of ruthenocene with Cp and naphthalene ligands and a second bulky pyridine ligand
- ↑ Labonne, Aurélie; Kribber, Thomas; Hintermann, Lukas (2006). "Highly Active in Situ Catalysts for Anti-Markovnikov Hydration of Terminal Alkynes". Organic Letters. 8 (25): 5853–6. doi:10.1021/ol062455k. PMID 17134289.
- ↑ Nishizawa, Mugio; Asai, Yumiko; Imagawa, Hiroshi (2006). "TiCl4Induced Anti-Markovnikov Rearrangement". Organic Letters. 8 (25): 5793–6. doi:10.1021/ol062337x. PMID 17134274..