Long-chain-fatty-acid—CoA ligase
| long-chain-fatty-acid—CoA ligase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Long chain fatty acyl-CoA synthetase homodimer from Thermus thermophilus.[1] | |||||||||
| Identifiers | |||||||||
| EC number | 6.2.1.3 | ||||||||
| CAS number | 9013-18-7 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
| acyl-CoA synthetase long-chain family member 1 | |
|---|---|
| Identifiers | |
| Symbol | ACSL1 |
| Alt. symbols | FACL2 |
| Entrez | 2180 |
| HUGO | 3569 |
| OMIM | 152425 |
| RefSeq | NM_001995 |
| UniProt | P33121 |
| Other data | |
| EC number | 6.2.1.3 |
| Locus | Chr. 4 q35 |
The long chain fatty acyl-CoA ligase (or synthetase) is an enzyme of the ligase family that activates the breakdown of complex fatty acids.[2] Long chain fatty acyl-CoA synthetase catalyzes the formation of fatty acyl-CoA by a two-step process proceeding through an adenylated intermediate.[3] The enzyme catalyzes the following reaction:
- Fatty acid + CoA + ATP ⇌ Acyl-CoA + AMP + PPi
It is present in all organisms from bacteria to humans. It catalyzes the pre-step reaction for β-oxidation of fatty acids or can be incorporated in phospholipids.
Function
Long chain fatty acyl-CoA synthetase, LC-FACS, plays a role in the physiological regulation of various cellular functions via the production of long chain fatty acyl-CoA esters, which reportedly have affected protein transport, enzyme activation, protein acylation, cell signaling, and transcriptional regulation.[1] The formation of fatty acyl-CoA is catalyzed in two steps: a stable intermediate of fatty acyl-AMP molecule and then the product is formed—fatty acid acyl-CoA molecule.[4]
Fatty acyl CoA synthetase catalyzes the activation of a long fatty acid chain to a fatty acyl CoA, requiring the energy of 1 ATP to AMP and pyrophosphate. This step uses 2 "ATP equivalents" because pyrophosphate is cleaved into 2 molecules of inorganic phosphate, breaking a high-energy phosphate bond.
Mechanism and active site
The mechanism for Long Chain Fatty Acyl-CoA Synthetase is a “bi uni uni bi ping-pong” mechanism.[1] The uni and bi prefixes refer to the number of substrates that enter the enzyme and the number of products that leave the enzyme; bi describes a situation where two substrates enter the enzyme at the same time. Ping-pong signifies that a product is released before another substrate can bind to the enzyme.
In step one, ATP and a long chain fatty acid enter the enzyme’s active site. Within the active site the negatively charged oxygen on the fatty acid attacks the alpha phosphate on ATP, forming an ATP-long chain fatty acid intermediate. (Step 1, Figure 3) In the second step, Pyrophosphate (PPi) leaves, resulting in an AMP-long chain fatty acid molecule within the enzyme’s active site. (Step 2, Figure 3) Coenzyme A now enters the enzyme and another intermediate is formed which consists of AMP-long chain fatty acid-Coenzyme A. (Step 3, Figure 3) At the end of this mechanism two products are released, AMP and acyl coa synthetase. (Step 4, Figure 3)
Acyl CoA is formed from long chain fatty acids through an acyl substitution. In an ATP dependent reaction, the fatty acid carboxylate is converted to a thioester. The final products of this reaction are acyl-CoA, pyrophosphate (PPi) and AMP.

Structure
There are several highly conserved areas and a 20-30% amino acid sequence similarity between the members of this superfamily.[1] The enzymes in the family consist of a large N-terminal and a small C-terminal domain, with the catalytic site positioned between the two domains.[1] Substrate binding may affect the relative positions of the C- and N-terminal domains. The C-terminal domain of LC-FACS is assumed to be in an open conformation when a substrate is absent and in a closed conformation when a substrate is bound.[1] The accessibility of the active site to solvent is reduced when the C- and N-terminal domains approach one another.[5]
The structure-function relationship between LC-FACS and the formation and processing of the acyl-AMP intermediate was still unclear. A domain swapped dimer is formed by LC-FACS, with monomer interacting at the N-terminal domains.[6] A large electrostatically positive concave is located at the back of the structure in the central valley of the homodimer.[1] Asp15 forms an intermolecular salt bridge with Arg176 in the dimer interactions. An intermolecular hydrogen bond is formed between the main chain carbonyl group of Glu16and the side chain of Arg199. At the interface, Glu175 forms an intermolecular salt bridge with Arg199.[5][7][8][9] The L motif, a six-amino acid peptide linker, connects the large N-terminal domain and a small C-terminal domain of each LC-FACS monomer.[1] The N-terminal domain is composed of two subdomains: a distorted antiparallel β-barrel and two β-sheets surrounded by α-helices forming an αβαβα sandwich.[1] The small C-terminal globular domain consists of two-stranded β-sheet and a three-stranded antiparallel β-sheet flanked by three α-helices.[1]
Dimer interaction

The dimerization of LC-FACS is stabilized through a salt bridge between Asp15 of sequence A and Arg176 of sequence B. Figure 4 shows this salt bridge between these two amino acids. The yellow line between Asp15 and Arg176 shows the salt bridge present.
ATP binding to the C-terminal domain
The conformations of the C-teriminal domain of the LC-FACS structures are dependent on the presence of a ligand.[1] AMP-PNP, a nonhydrolyzable ATP analogue, bound to LC-FACS results in the closed conformation with the C- and N-terminal domains directly interacting.[1] In crystal structures, AMP-PNP is bound in a crevasse of each monomer at the interface between the N- and C-terminal domains.[1] The closed conformation of the C-terminal domain is retained with myristroyl-AMP.[1] Three residues in the C-terminal domain, Glu443, Glu475, and Lys527, interact noncovalently with L motif residues and the N-terminal domain to stabilize the closed conformation.[1] There are two types of open conformations in the C-terminal domains of the uncomplexed structure. The C- and N-terminal domains do not interact directly for both monomers of the dimer.[1] An extensive hydrogen bond network is used by the AMP moiety of the bound ATP molecule to hold the C- and N-terminal domains together.[1]

Fatty acid-binding tunnel
Bulkier long chain fatty acids are bound by a fatty acid-binding tunnel that is located in the N-terminal domain of each monomer.[1] A large β-sheet and an α-helix cluster surround the tunnel which extends from the concave cavity in the central valley to the site of ATP-binding.[1] There are two distinct paths in the large central pathway of the tunnel in the complex structure, which includes the “ATP path” and the “center path,” separated by the indole ring of Trp234 in the G motif.[1] There is also another branch of the central pathway known as the “dead and branch.” The indole ring of Trp234 closes the fatty acid-binding tunnel in the uncomplexed structure.[1] It opens up once AMP-PNP binds through hydrogen bond formation between β-phosphate and the nitrogen on the ring of Trp234.[1] During this time, the closed conformation is adopted by the mobile C-terminal domain. There is a shift in the flexible loop of the G motif in the closed structures of LC-FACS, resulting in a wider dead end branch compared to the uncomplexed forms.[1]
The ATP binding site is connected to an ATP path that is a hydrophobic channel in the fatty acid-binding tunnel.[1] The fatty acid enters through the center path extending from the interface of the dimer along β-strand 13 to the ATP path.[1] The connection between the two paths is blocked by the indole ring of Trp234 in the absence of ATP. Water molecules fill the center path in the AMP-PNP and myristoyl-AMP complex structures and through the entrance of the center path, they connect to the bulk solvent regions. The basic residues from each monomer, Lys219, Arg296, Arg297, Arg321, Lys350, and Lys 354, cause the entrance of the center path to generate a positive electrostatic potential.[1] The dead end branch contains residues 235-243 and extends from the fatty acid-binding tunnel to α-helix h.[1] The bottom of the dead end branch consists of a hydrophilic environment from the water molecules and polar side chains.[1]
Domains
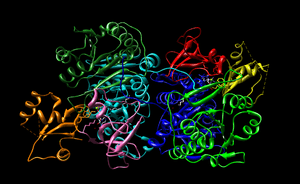
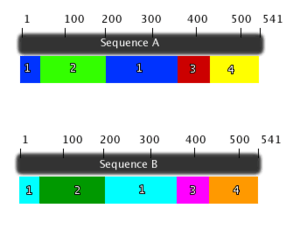
The domains founds in Long chain fatty acyl CoA synthetase are shown both in the enzyme view (figure 6) and sequence view (figure 7). LC-FACS has five domains. After searching 1v26 in Entrez, the location of the 5 domains was shown and was used to create figure 6 and 7. The ribbons colors in figure 6 correspond to the colors of the figure 7.
Inhibition by long chain fatty acyl-CoAs
A long term and short term regulation controls fatty acid synthesis.[4] Long term fatty acid synthesis regulation is dependent on the rate of acetyl-CoA carboxylase (ACC) synthesis, the rate-limiting enzyme and first enzyme of the fatty acid synthesis, and fatty acid synthase (FAS), the second and major enzyme of the fatty acid synthesis.[4][10][11][12] Cellular fatty acyl-CoA is involved in the short term regulation, but there is not a full understanding of the mechanisms.[13]
Free fatty acids inhibits the de novo fatty acid synthesis and appears to be dependent on the formation of long chain fatty acyl-CoAs.[14] Studies have shown that long chain fatty acyl-CoAs inhibit ACC and FAS via feedback inhibition.[15][16][17][18] Long chain fatty acyl-CoA’s inhibitory effect on the fatty acid synthesis may be a result of its regulation of lipogenic enzymes in a feedback manner through gene transcription suppression.[19]
Long-chain fatty-acid-CoA ligase in cells catalytically synthesizes long chain fatty acyl-CoAs. Long-chain fatty-acid-CoA ligase may be involved in an important role in the suppression of fatty acid synthesis and it has been reported that it played a part in fatty acid synthesis inhibition.[20] It was recently found that vitamin D3 upregulates FACL3, which forms long-chain fatty acid synthesis through the use of myristic acid, eicosapentaenoic acid (EPA), and arachidonic acid as substrates, in expression and activity levels.[21] FACL3 contributes to vitamin D3 growth inhibitory effect in human prostate cancer LNCaP cells.[21] A current study reports that the feedback inhibition of FAS expression by long chain fatty acyl-CoAs causes the downregulation of FAS mRNA by vitamin D3.[4][22]
Diseases
Adrenoleukodystrophy (ALD), is the build up of long chain fatty acids in the brain and adrenal cortex, because of the decreased activity of long chain fatty acyl coa synthetase.[23] The oxidation of the long chain fatty acids normally occurs in the peroxisome where the long chain fatty acyl coa synthetase is found. Long chain fatty acids enter the peroxisome via a transporter protein, ALDP, which creates a gate in the membrane of the peroxisome. In ALD the gene for this peroximal membrane transporter, ALDP, is defective, preventing long chain fatty acids from entering the peroxisome.[24]
Examples
Human genes encoding long-chain-fatty-acid—CoA ligase enzymes include:
See also
- Fatty acyl-CoA synthase
- Triacsin C - an inhibitor of Fatty acyl CoA synthetase
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 PDB: 1V26; Hisanaga Y, Ago H, Nakagawa N, Hamada K, Ida K, Yamamoto M, Hori T, Arii Y, Sugahara M, Kuramitsu S, Yokoyama S, Miyano M (July 2004). "Structural basis of the substrate-specific two-step catalysis of long chain fatty acyl-CoA synthetase dimer". J. Biol. Chem. 279 (30): 31717–26. doi:10.1074/jbc.M400100200. PMID 15145952.
- ↑ Soupene E, Kuypers FA (May 2008). "Mammalian long-chain acyl-CoA synthetases". Exp. Biol. Med. (Maywood). 233 (5): 507–21. doi:10.3181/0710-MR-287. PMC 3377585
 . PMID 18375835.
. PMID 18375835. - ↑ Bækdal T, Schjerling CK, Hansen JK, Knudsen J (1997). "Analysis of long-chain acyl-Coenzyme A esters". In Christie W. Advances in Lipid Methodology (Three ed.). Ayr, Scotland: Oily Press. pp. 109–131. ISBN 0-9514171-7-7.
- 1 2 3 4 Qiao S, Tuohimaa P (November 2004). "Vitamin D3 inhibits fatty acid synthase expression by stimulating the expression of long-chain fatty-acid-CoA ligase 3 in prostate cancer cells". FEBS Lett. 577 (3): 451–4. doi:10.1016/j.febslet.2004.10.044. PMID 15556626.
- 1 2 Conti E, Stachelhaus T, Marahiel MA, Brick P (July 1997). "Structural basis for the activation of phenylalanine in the non-ribosomal biosynthesis of gramicidin S". EMBO J. 16 (14): 4174–83. doi:10.1093/emboj/16.14.4174. PMC 1170043
 . PMID 9250661.
. PMID 9250661. - ↑ Liu Y, Eisenberg D (June 2002). "3D domain swapping: as domains continue to swap". Protein Sci. 11 (6): 1285–99. doi:10.1110/ps.0201402. PMC 2373619
 . PMID 12021428.
. PMID 12021428. - ↑ Conti E, Franks NP, Brick P (March 1996). "Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes". Structure. 4 (3): 287–98. doi:10.1016/S0969-2126(96)00033-0. PMID 8805533.
- ↑ May JJ, Kessler N, Marahiel MA, Stubbs MT (September 2002). "Crystal structure of DhbE, an archetype for aryl acid activating domains of modular nonribosomal peptide synthetases". Proc. Natl. Acad. Sci. U.S.A. 99 (19): 12120–5. doi:10.1073/pnas.182156699. PMC 129408
 . PMID 12221282.
. PMID 12221282. - ↑ Gulick AM, Starai VJ, Horswill AR, Homick KM, Escalante-Semerena JC (March 2003). "The 1.75 A crystal structure of acetyl-CoA synthetase bound to adenosine-5'-propylphosphate and coenzyme A". Biochemistry. 42 (10): 2866–73. doi:10.1021/bi0271603. PMID 12627952.
- ↑ Burton DN, Collins JM, Kennan AL, Porter JW (August 1969). "The effects of nutritional and hormonal factors on the fatty acid synthetase level of rat liver". J. Biol. Chem. 244 (16): 4510–6. PMID 5806590.
- ↑ Craig MC, Dugan RE, Muesing RA, Slakey LL, Porter JW (July 1972). "Comparative effects of dietary regimens on the levels of enzymes regulating the synthesis of fatty acids and cholesterol in rat liver". Arch. Biochem. Biophys. 151 (1): 128–36. doi:10.1016/0003-9861(72)90481-X. PMID 5044513.
- ↑ Majerus PW, Kilburn E (November 1969). "Acetyl coenzyme A carboxylase. The roles of synthesis and degradation in regulation of enzyme levels in rat liver". J. Biol. Chem. 244 (22): 6254–62. PMID 4981792.
- ↑ Goodridge AG (June 1973). "Regulation of fatty acid synthesis in isolated hepatocytes. Evidence for a physiological role for long chain fatty acyl coenzyme A and citrate". J. Biol. Chem. 248 (12): 4318–26. PMID 4145797.
- ↑ McGee R, Spector AA (July 1975). "Fatty acid biosynthesis in Erlich cells. The mechanism of short term control by exogenous free fatty acids". J. Biol. Chem. 250 (14): 5419–25. PMID 237919.
- ↑ Guynn RW, Veloso D, Veech RL (November 1972). "The concentration of malonyl-coenzyme A and the control of fatty acid synthesis in vivo". J. Biol. Chem. 247 (22): 7325–31. PMID 4638549.
- ↑ Numa S, Ringelmann E, Lynen F (December 1965). "[On inhibition of acetyl-CoA-carboxylase by fatty acid-coenzyme A compounds]". Biochem Z (in German). 343 (3): 243–57. PMID 5875764.
- ↑ Goodridge AG (November 1972). "Regulation of the activity of acetyl coenzyme A carboxylase by palmitoyl coenzyme A and citrate". J. Biol. Chem. 247 (21): 6946–52. PMID 5082134.
- ↑ Sumper M, Träuble H (February 1973). "Membranes as acceptors for palmitoyl CoA in fatty acid biosynthesis". FEBS Lett. 30 (1): 29–34. doi:10.1016/0014-5793(73)80612-X. PMID 11947055.
- ↑ Faergeman NJ, Knudsen J (April 1997). "Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling". Biochem. J. 323 (1): 1–12. PMC 1218279
 . PMID 9173866.
. PMID 9173866. - ↑ Fox SR, Hill LM, Rawsthorne S, Hills MJ (December 2000). "Inhibition of the glucose-6-phosphate transporter in oilseed rape (Brassica napus L.) plastids by acyl-CoA thioesters reduces fatty acid synthesis". Biochem. J. 352 (2): 525–32. doi:10.1042/0264-6021:3520525. PMC 1221485
 . PMID 11085947.
. PMID 11085947. - 1 2 Qiao S, Tuohimaa P (June 2004). "The role of long-chain fatty-acid-CoA ligase 3 in vitamin D3 and androgen control of prostate cancer LNCaP cell growth". Biochem. Biophys. Res. Commun. 319 (2): 358–68. doi:10.1016/j.bbrc.2004.05.014. PMID 15178414.
- ↑ Qiao S, Pennanen P, Nazarova N, Lou YR, Tuohimaa P (May 2003). "Inhibition of fatty acid synthase expression by 1alpha,25-dihydroxyvitamin D3 in prostate cancer cells". J. Steroid Biochem. Mol. Biol. 85 (1): 1–8. doi:10.1016/S0960-0760(03)00142-0. PMID 12798352.
- ↑ "Adrenoleukodystrophy Information Page". National Institute of Neurological Disorders and Stroke (NINDS). 2009-03-18. Retrieved 2010-01-16.
- ↑ Kemp S, Watkins P (2009-03-03). "very long-chain fatty acids and X-ALD". X-linked Adrenoleukodystrophy Database. Archived from the original on December 21, 2009. Retrieved 2010-01-16.
External links
- ACSL6 protein, human at the US National Library of Medicine Medical Subject Headings (MeSH)