Liothyronine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cytomel |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682462 |
| Pregnancy category |
|
| ATC code | H03AA02 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 99.7% |
| Biological half-life | 2.5 days |
| Identifiers | |
| |
| CAS Number |
6893-02-3 |
| PubChem (CID) | 16218759 |
| IUPHAR/BPS | 2634 |
| DrugBank |
DB00279 |
| ChemSpider |
17346129 |
| UNII |
06LU7C9H1V |
| ChEBI |
CHEBI:6484 |
| ChEMBL |
CHEMBL1201119 |
| PDB ligand ID | T3 (PDBe, RCSB PDB) |
| ECHA InfoCard | 100.000.203 |
| Chemical and physical data | |
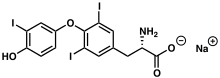
| Formula | C15H11I3NNaO4 |
| Molar mass | 672.96 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Liothyronine is a synthetic form of thyroid hormone (T3) used to treat hypothyroidism and myxedema coma. It is also used as an augmentation strategy in treating Major Depressive Disorder when used in combination with antidepressants.[1] It is marketed as the sodium salt under the brand name Cytomel (or Tertroxin in Australia).
Uses
Physicians can use this instead of or in addition to levothyroxine (T4) for patients undergoing thyroid withdrawal. When a patient has thyroid cancer or Graves' disease, ablation therapy with radioactive iodine (131I) can be used to remove any trace thyroid tissue. For 131I therapy to be effective, the trace thyroid tissue must be avid to iodine. The best method is to starve the tissue of iodine but this can lead to hypothyroid symptoms for the patient. Withdrawal from levothyroxine can be done but it takes six weeks of withdrawal for the remaining thyroid tissue to be completely starved. Six weeks is needed owing to levothyroxine's long half life. Six weeks can be inconvenient for the patient and delay treatment. Liothyronine instead can be taken and withdrawn from for two weeks to starve the thyroid tissue. This is much safer and more convenient than levothyroxine. Also, due to its quicker onset of action when compared to levothyroxine, it sometimes is a better option in patients with myxedema coma or in patients preparing for 131I therapy for thyroid cancer.[2]
Low-dose liothyronine has been shown to improve depression symptoms in patients with normal thyroid function who do not have adequate relief from their depression after trying several different antidepressants. When added to existing medications, liothyronine helped achieve remission in 24% of patients participating in a subset of the large STAR*D depression trial.[3] According to a 2001 meta-analysis that analyzed the effectiveness of adding Liothyronine to tricyclic antidepressants, women in particular may benefit from Liothyronine.[4] The average effective dose for depression was 45 mcg of Liothyronine daily, which is lower than the doses used for treating hypothyroidism.[3] About 9% of patients stopped taking liothyronine due to side effects.[3] The difference in gender response may be due to differences in metabolism of thyroid precursors.[5]
An algorithm developed from the STAR*D trial recommends liothyronine as an option when patients have failed two antidepressant medications.[6][7]
Advantages in thyroid hormone replacement
See Hypothyroidism for an in-depth explanation of hormone replacement.
Liothyronine is an option for routine thyroid hormone replacement. It has a half-life of 24 hours. [8] (although the stated biological half-life is 2.5 days). This compares to a half life of 7 days with levothyroxine. The shorter half-life allows patients to know if they are taking too much (indicated by a heart rate>100 bpm for more than 24 hours) or if they should take more (no increase in energy). It is recommended that labs be drawn monthly and the dose increased until the patients hypothyroid symptoms resolve.
Per the liothyronine product insert, the starting dose may start at 5 mcg daily and increase by 5 mcg every two weeks. However, it should be noted that liothyronine should be taken with levothyroxine when liothyronine is taken long-term to avoid potential heart rate and rhythm abnormalities. Taking both levothyhroxine and liothyronine separately allows for optimizing of reverse T3 and resolution of potential conversion problems of T4 to T3. Peripheral conversion problems are common when there is physiological stress (this will be felt as increased fatigue).
Desiccated thyroid products have both T4 (levothyroxine) and T3 (liothyronine) in one tablet in a ratio of 4 to 1 (T4 to T3). If the body does not metabolize thyroid hormones in this ratio (which is possible due to a thyroid problem) symptoms may worsen. This may be a reason to take levothyroxine and liothyronine separately and target for upper range for fT3 and fT4, with rT3 in the bottom quartile. This is one therapeutic approach for patients who feel worse (HR>100bpm or profound fatigue) after they start either levothyroxine or a desiccated thyroid product. These patients may benefit more from a customized ratio of T3/T4 such as reducing the ratio from 4:1 to 3:1 to 2:1 and so on until both symptomatic improvement and labs in ideal range as explained above. Patients who still have complications after obtaining normal labs may benefit from a slow-release formulation from a compounded pharmacy (no slow-release thyroid product is currently manufactured so it must be compounded).
Contraindications
Any person with a hypersensitivity to liothyronine sodium or any active ingredient of the formulation should not be on this medication. If there is uncorrected adrenal insufficiency or thyrotoxicosis, a different approach to therapy must be considered.[9]
Side effects
Liothyronine may cause a number of side effects, mostly similar to symptoms of hyperthyroidism, which include:[10]
- weight loss
- tremor
- headache
- upset stomach
- vomiting
- diarrhea
- stomach cramps
- nervousness
- irritability
- insomnia
- excessive sweating
- increased appetite
- fever
- changes in menstrual cycle
- sensitivity to heat
Boxed warning
The package insert for Cytomel contains the following boxed warning, as do all thyroid hormones:[11]
Drugs with thyroid hormone activity, alone or together with other therapeutic agents, have been used for the treatment of obesity. In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction. Larger doses may produce serious or even life-threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects.
Safety considerations
Pregnancy Per the U.S. FDA, liothyronine is categorized as Pregnancy Category A.[9] Thyroid hormone is minimally transferred to the fetus or placenta, however as of October 2014, studies have not shown any adverse effects to the fetus. Hypothyroid mothers should continue to take thyroid hormone replacement therapy throughout pregnancy to avoid adverse events.[12][13]
Nursing Breastmilk contains a low amount of thyroid hormone, so it is important to exercise caution when breastfeeding while taking liothyronine.[12]
Elderly Elderly patients should be started on lower doses of liothyronine.[9] Plasma (T3) concentrations in this population are decreased by 25% to 40%.[12] TSH must be routinely monitored since there is a risk of coronary artery disease, hyperthyroidism and excessive bone loss from inadequate or abnormal thyroid replacement.[12]
Pharmacology
Liothyronine is the most potent form of thyroid hormone. As a salt of triiodothyronine (T3), it is chemically similar and pharmacologically equivalent to T3. As such, it acts on the body to increase the basal metabolic rate, affect protein synthesis and increase the body's sensitivity to catecholamines (such as adrenaline) by permissiveness. As monotherapy or in combination therapy with SSRIs, liothyronine may also enhance generation of new neurons in the central nervous system.[1] The thyroid hormones are essential to proper development and differentiation of all cells of the human body. These hormones also regulate protein, fat, and carbohydrate metabolism, affecting how human cells use energetic compounds.
In comparison to levothyroxine (T4), liothyronine has a faster onset of action as well as a shorter biological half-life, which may be due to less plasma protein binding to thyroxine-binding globulin and transthyretin.
See also
References
- 1 2 "The American Journal of Psychiatry". Retrieved 29 Oct 2014.
- ↑ http://pharmacistanswers.com/hypothyroidism-medications.html
- 1 2 3 Nierenberg, AA; Fava, M; Trivedi, MH; Wisniewski, SR; Thase, ME; McGrath, PJ; Alpert, JE; Warden, D; Luther, JF; Niederehe, G; Lebowitz, B; Shores-Wilson, K; Rush, AJ (September 2006). [http://ajp.psychiatryonline.org.ucsf.idm.oclc.org/data/Journals/AJP/3775/06aj1519.PDF>. "A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report."] (PDF). The American Journal of Psychiatry. 163 (9): 1519–30; quiz 1665. doi:10.1176/appi.ajp.163.9.1519. PMID 16946176. Retrieved 1 November 2014.
- ↑ Altshuler, LL; Bauer, M; Frye, MA; Gitlin, MJ; Mintz, J; Szuba, MP; Leight, KL; Whybrow, PC (October 2001). "Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature.". The American Journal of Psychiatry. 158 (10): 1617–22. doi:10.1176/appi.ajp.158.10.1617. PMID 11578993.
- ↑ Berent, Dominika; Zboralski, Krzysztof; Orzechowska, Agata; Gałecki, Piotr (18 January 2014). "Thyroid hormones association with depression severity and clinical outcome in patients with major depressive disorder". Molecular Biology Reports. 41 (4): 2419–2425. doi:10.1007/s11033-014-3097-6.
- ↑ Cooper-Kazaz, R; Cohen, R; Karagichev, L; Muhammed-Moussa, S; Grupper, D; Drori, T; Newman, ME; Sackeim, HA; Glaser, B; Lerer, B (2007). "Combined treatment with sertraline and liothyronine in major depression: a randomized, double-blind, placebo-controlled trial.". Arch Gen Psychiatry. 64: 679–688. doi:10.1001/archpsyc.64.6.679. PMID 17548749.
- ↑ G, Bradley; Rush, J; Trivedi, M; Wisniewski, S; Spencer, D; Fava, M (2008). "The STAR*D Study: Treating Depression in the Real World.". Cleveland Clinic Journal of Medicine. 75.1: 57–66. doi:10.3949/ccjm.75.1.57.
- ↑ Koda-Kimble, MA; Alldredge, BK (2012). Applied therapeutics: the clinical use of drugs (10th ed.). Baltimore: Wolters Kluwer Health/Lippincott Williams & Wilkins. ISBN 978-1609137137.
- 1 2 3 Cytomel (Liothyronine Sodium) Drug Information: Warnings and Precautions - Prescribing Information at RxList, retrieved on 29-October-2014
- ↑ MedlinePlus. "Liothyronine." Last accessed July 14, 2007.
- ↑ Cytomel (Liothyronine Sodium) Drug Information: Warnings and Precautions - Prescribing Information at RxList, retrieved on 14-April-2014
- 1 2 3 4 "Liothyronine (Lexi-Drugs)". LexiComp. Retrieved 29 October 2014.
- ↑ Montalvo, JM; Wahner, HW; Mayberry, WE; Lum, RK (Aug 1973). "Serum triiodothyronine, total thyroxine, and thyroxine to triiodothyronine ratios in paired maternal-cord sera and at one week and one month of age.". Pediatr Res. 7: 706–711. doi:10.1203/00006450-197308000-00006. PMID 4200034.