Ion-exchange membranes
Ion-exchange membranes transport dissolved ions across a conductive polymeric membrane.[1] The membranes are often used in desalination and chemical recovery applications, moving ions from one solution to another with little passage of water.[2]
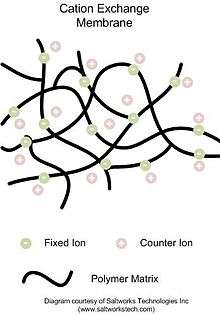
Ion-exchange membranes are made of a polymeric material attached to charged ion groups. Anion-exchange membranes contain fixed cationic groups with predominantly mobile anions; because anions are the majority species, most of the conductivity is due to anion transport. Cation-exchange membranes contain fixed anionic groups with predominantly mobile cations; because cations are the majority species, most of the conductivity is due to cation transport. The selectivity of the membranes is due to Donnan equilibrium and Donnan exclusion and not due to physically blocking or electrostatically excluding specific charged species.
There are two primary classes of membranes: heterogeneous and homogeneous. Heterogeneous membranes have low cost and a thicker composition with higher resistance and a rough surface that can be subject to fouling. Homogeneous membranes are more expensive, but have a thinner composition with lower resistance and a smooth surface, less susceptible to fouling. Homogeneous membrane surfaces can be modified to alter the membrane permselectivity to protons, monovalent ions, and divalent ions.[3]
The permselectivity of ion-exchange membranes describes their charge selectivity. This charge selectivity reflects the membrane’s ability to discriminate between ions of opposite charge. A higher selectivity leads to increased recovery and performance of the membrane.[3]
Applications
Ion-exchange membranes are traditionally used in electrodialysis or diffusion dialysis by means of an electrical potential or concentration gradient, respectively, to selectivity transport cationic and anionic species. When applied in an electrodialysis desalination process, anion- and cation-exchange membranes are typically arranged in an alternating pattern between two electrodes (an anode and a cathode) within the electrodialysis stack. A galvanic potential is supplied as a voltage generated at the electrodes.[3]
A typical industrial electrodialysis stack consists of two chambers: a product-water chamber and a concentrate-reject chamber. During stack operation, salts are transferred from the product to the concentrate. As a result, the reject stream is concentrated up while the product stream is desalted.[3]
Exemplary applications of ion-exchange membranes utilized in electrodialysis and EDR include seawater desalination, industrial wastewater treatment of highly scaling waters, food and beverage production, and other industrial wastewaters.[3]
References
- ↑ Tanaka, Yoshinobu (January 2015). Ion exchange membranes: fundamentals and applications. Japan: Elsevier. p. 47. ISBN 978-0-444-63319-4.
- ↑ Strathmann, Heiner (2004). Membrane Science and Technology Series, 9: Ion Exchange Membrane Separation Processes (First ed.). San Diego, Ca, USA: Elsevier. pp. 90–206. ISBN 0-444-50236-X.
- 1 2 3 4 5 Davis, T. S. (1990). "Electrodialysis", in Handbook of Industrial Membrane Technology (First ed.). New Jersey, USA: Noyes Publication. pp. 40–102. ISBN 9780815512059.