Insular cortex
| Insular cortex | |
|---|---|
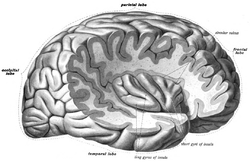 The insula of the right side, exposed by removing the opercula. | |
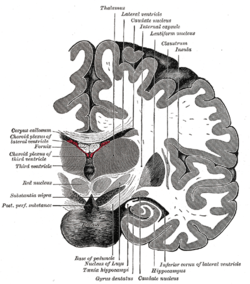 Coronal section of brain immediately in front of pons. (Insula labeled at upper right.) | |
| Details | |
| Artery | Middle cerebral |
| Identifiers | |
| Latin | Cortex insularis |
| NeuroNames | hier-93 |
| NeuroLex ID | Insula |
| TA | A14.1.09.149 |
| FMA | 67329 |
In each hemisphere of the mammalian brain the insular cortex (often called insula, or insular lobe) is a portion of the cerebral cortex folded deep within the lateral sulcus (the fissure separating the temporal lobe from the parietal and frontal lobes).
The insulae are believed to be involved in consciousness and play a role in diverse functions usually linked to emotion or the regulation of the body's homeostasis. These functions include perception, motor control, self-awareness, cognitive functioning, and interpersonal experience. In relation to these, it is involved in psychopathology.
The insular cortex is divided into two parts: the larger anterior insula and the smaller posterior insula in which more than a dozen field areas have been identified. The cortical area overlying the insula toward the lateral surface of the brain is the operculum (meaning lid). The opercula are formed from parts of the enclosing frontal, temporal, and parietal lobes.
Structure
Connections
The anterior part of the insula is subdivided by shallow sulci into three or four short gyri.
The anterior insula receives a direct projection from the basal part of the ventral medial nucleus of the thalamus and a particularly large input from the central nucleus of the amygdala. In addition, the anterior insula itself projects to the amygdala.
One study on rhesus monkeys revealed widespread reciprocal connections between the insular cortex and almost all subnuclei of the amygdaloid complex. The posterior insula projects predominantly to the dorsal aspect of the lateral and to the central amygdaloid nuclei. In contrast, the anterior insula projects to the anterior amygdaloid area as well as the medial, the cortical, the accessory basal magnocellular, the medial basal, and the lateral amygdaloid nuclei.[1]
The posterior part of the insula is formed by a long gyrus.
The posterior insula connects reciprocally with the secondary somatosensory cortex and receives input from spinothalamically activated ventral posterior inferior thalamic nuclei. It has also been shown that this region receives inputs from the ventromedial nucleus (posterior part) of the thalamus that are highly specialized to convey homeostatic information such as pain, temperature, itch, local oxygen status, and sensual touch.[2]
A human neuroimaging study using diffusion tensor imaging revealed that the anterior insula is interconnected to regions in the temporal and occipital lobe, opercular and orbitofrontal cortex, triangular and opercular parts of the inferior frontal gyrus. The same study revealed differences in the anatomical connection patterns between the left and right hemisphere.[3]
The 'circular sulcus of insula' (or sulcus of Reil[4])is a semi-circular sulcus or fissure[4] that separates the insula from the neighboring gyri of the operculum[5] in the front, above, and behind.[4]
Cytoarchitecture
The insular cortex has regions of variable cell structure or cytoarchitecture, changing from granular in the posterior portion to agranular in the anterior portion. The insula also receives differential cortical and thalamic input along its length. John Allman and his colleagues have shown that the anterior insular cortex contains a population of neurons, called spindle neurons. These are also called von Economo neurons, identified as characterising a distinctive subregion as the agranular frontal insula.[6]
Development
The insular cortex is considered a separate lobe of the telencephalon by some authorities.[7] Other sources see the insula as a part of the temporal lobe.[8] It is also sometimes grouped with limbic structures deep in the brain into a limbic lobe. As a paralimbic cortex, the insular cortex is considered to be a relatively old structure.
Function
Multimodal Sensory Processing, Sensory Binding
Functional imaging studies show activation of the insula during audio-visual integration tasks[9][10]
Interoceptive awareness
There is evidence that, in addition to its base functions, the insula may play a role in certain "higher" functions that operate only in humans and great apes. The spindle neurons found at a higher density in the right frontal insular cortex are also found in the anterior cingulate cortex, which is another region that has reached a high level of specialization in great apes. It has been speculated that these neurons are involved in cognitive-emotional processes that are specific to primates including great apes, such as empathy and metacognitive emotional feelings. This is supported by functional imaging results showing that the structure and function of the right frontal insula is correlated with the ability to feel one's own heartbeat, or to empathize with the pain of others. It is thought that these functions are not distinct from the "lower" functions of the insula but rather arise as a consequence of the role of the insula in conveying homeostatic information to consciousness.[11][12] The right anterior insula aids interoceptive awareness of body states, such as the ability to time one's own heartbeat. Moreover, greater right anterior insular gray matter volume correlates with increased accuracy in this subjective sense of the inner body, and with negative emotional experience.[13] It is also involved in the control of blood pressure,[14] in particular during and after exercise,[14] and its activity varies with the amount of effort a person believes he/she is exerting.[15][16]
The insular cortex also is where the sensation of pain is judged as to its degree.[17] Further, the insula is where a person imagines pain when looking at images of painful events while thinking about their happening to one's own body.[18] Those with irritable bowel syndrome have abnormal processing of visceral pain in the insular cortex related to dysfunctional inhibition of pain within the brain.[19]
Another perception of the right anterior insula is the degree of nonpainful warmth[20] or nonpainful coldness[21] of a skin sensation. Other internal sensations processed by the insula include stomach or abdominal distension.[22][23] A full bladder also activates the insular cortex.[24]
One brain imaging study suggests that the unpleasantness of subjectively perceived dyspnea is processed in the right human anterior insula and amygdala.[25]
The cerebral cortex processing vestibular sensations extends into the insula,[26] with small lesions in the anterior insular cortex being able to cause loss of balance and vertigo.[27]
Other noninteroceptive perceptions include passive listening to music,[28] laughter, and crying,[29] empathy and compassion,[30] and language.[31]
Motor control
In motor control, it contributes to hand-and-eye motor movement,[32][33] swallowing,[34] gastric motility,[35] and speech articulation.[36][37] It has been identified as a "central command” centre that ensures that heart rate and blood pressure increase at the onset of exercise.[38] Research upon conversation links it to the capacity for long and complex spoken sentences.[39] It is also involved in motor learning[40] and has been identified as playing a role in the motor recovery from stroke.[41]
Homeostasis
It plays a role in a variety of homeostatic functions related to basic survival needs, such as taste, visceral sensation, and autonomic control. The insula controls autonomic functions through the regulation of the sympathetic and parasympathetic systems.[42][43] It has a role in regulating the immune system.[44][45][46]
Self
It has been identified as playing a role in the experience of bodily self-awareness,[47][48] sense of agency,[49] and sense body ownership.[50]
Social emotions
The anterior insula processes a person's sense of disgust both to smells[51] and to the sight of contamination and mutilation[52] — even when just imagining the experience.[53] This associates with a mirror neuron-like link between external and internal experiences.
In social experience, it is involved in the processing of norm violations,[54] emotional processing,[55] empathy,[56] and orgasms.[57]
The insula is active during social decision making. Tizianna Quarto et al. measured emotional intelligence (EI) (the ability to identify, regulate, and process emotions) of sixty-three healthy subjects. Using fMRI EI was measured in correlation with left insular activity. The subjects were shown various pictures of facial emotions and tasked with deciding to approach or avoid the person in the picture. The results of the social decision task yielded that individuals with high EI scores had left insular activation when processing fearful faces. Individuals with low EI scores had left insular activation when processing angry faces.[58]
Emotions
The insular cortex, in particular its most anterior portion, is considered a limbic-related cortex. The insula has increasingly become the focus of attention for its role in body representation and subjective emotional experience. In particular, Antonio Damasio has proposed that this region plays a role in mapping visceral states that are associated with emotional experience, giving rise to conscious feelings. This is in essence a neurobiological formulation of the ideas of William James, who first proposed that subjective emotional experience (i.e., feelings) arise from our brain's interpretation of bodily states that are elicited by emotional events. This is an example of embodied cognition.
In terms of function, the insula is believed to process convergent information to produce an emotionally relevant context for sensory experience. To be specific, the anterior insula is related more to olfactory, gustatory, vicero-autonomic, and limbic function, whereas the posterior insula is related more to auditory-somesthetic-skeletomotor function. Functional imaging experiments have revealed that the insula has an important role in pain experience and the experience of a number of basic emotions, including anger, fear, disgust, happiness, and sadness.[59]
The anterior insular cortex (AIC) is believed to be responsible for emotional feelings, including maternal and romantic love, anger, fear, sadness, happiness, sexual arousal, disgust, aversion, unfairness, inequity, indignation, uncertainty,[60] disbelief, social exclusion, trust, empathy, sculptural beauty, a ‘state of union with God’, and hallucinogenic state.[61]
Functional imaging studies have also implicated the insula in conscious desires, such as food craving and drug craving. What is common to all of these emotional states is that they each change the body in some way and are associated with highly salient subjective qualities. The insula is well-situated for the integration of information relating to bodily states into higher-order cognitive and emotional processes. The insula receives information from "homeostatic afferent" sensory pathways via the thalamus and sends output to a number of other limbic-related structures, such as the amygdala, the ventral striatum, and the orbitofrontal cortex, as well as to motor cortices.[62]
A study using magnetic resonance imaging found that the right anterior insula is significantly thicker in people that meditate.[63]
Another study using voxel-based morphometry and MRI on experienced Vipassana meditators was done to extend the findings of Lazar et al., which found increased grey matter concentrations in this and other areas of the brain in experienced meditators.[64]
Salience
Functional imaging research suggests the insula is involved in two types of salience. Interoceptive information processing that links interoception with emotional salience to generate a subjective representation of the body. This involves, first, the anterior insular cortex with the pregenual anterior cingulate cortex (Brodmann area 33) and the anterior and posterior mid-cingulate cortices, and, second, a general salience system concerned with environmental monitoring, response selection, and skeletomotor body orientation that involves all of the insular cortex and the mid-cingulate cortex.[65]
An alternative or perhaps complementary proposal is that the right anterior insular regulates the interaction between the salience of the selective attention created to achieve a task (the dorsal attention system) and the salience of arousal created to keep focused upon the relevant part of the environment (ventral attention system).[66] This regulation of salience might be particularly important during challenging tasks where attention might fatigue and so cause careless mistakes but if there is too much arousal it risks creating poor performance by turning into anxiety.[66]
Clinical significance
Progressive expressive aphasia
Progressive expressive aphasia is the deterioration of normal language function that causes individuals to lose the ability to communicate fluently while still being able to comprehend single words and intact other non-linguistic cognition. It is found in a variety of degenerative neurological conditions including Pick's disease, motor neuron disease, corticobasal degeneration, frontotemporal dementia, and Alzheimer's disease. It is associated with hypometabolism[67] and atrophy of the left anterior insular cortex.[68]
Addiction
A number of functional brain imaging studies have shown that the insular cortex is activated when drug abusers are exposed to environmental cues that trigger cravings. This has been shown for a variety of drugs, including cocaine, alcohol, opiates, and nicotine. Despite these findings, the insula has been ignored within the drug addiction literature, perhaps because it is not known to be a direct target of the mesotelencephalic dopamine system, which is central to current dopamine reward theories of addiction. Research published in 2007[69] has shown that cigarette smokers suffering damage to the insular cortex, from a stroke for instance, have their addiction to cigarettes practically eliminated. These individuals were found to be up to 136 times more likely to undergo a disruption of smoking addiction than smokers with damage in other areas. Disruption of addiction was evidenced by self-reported behavior changes such as quitting smoking less than one day after the brain injury, quitting smoking with great ease, not smoking again after quitting, and having no urge to resume smoking since quitting. The study was conducted on average eight years after the strokes, which opens up the possibility that recall bias could have affected the results.[70] More recent prospective studies, which overcome this limitation, have corroborated these findings[71][72] This suggests a significant role for the insular cortex in the neurological mechanisms underlying addiction to nicotine and other drugs, and would make this area of the brain a possible target for novel anti-addiction medication. In addition, this finding suggests that functions mediated by the insula, especially conscious feelings, may be particularly important for maintaining drug addiction, although this view is not represented in any modern research or reviews of the subject.[73]
A recent study in rats by Contreras et al.[74] corroborates these findings by showing that reversible inactivation of the insula disrupts amphetamine conditioned place preference, an animal model of cue-induced drug craving. In this study, insula inactivation also disrupted "malaise" responses to lithium chloride injection, suggesting that the representation of negative interoceptive states by the insula plays a role in addiction. However, in this same study, the conditioned place preference took place immediately after the injection of amphetamine, suggesting that it is the immediate, pleasurable interoceptive effects of amphetamine administration, rather than the delayed, aversive effects of amphetamine withdrawal that are represented within the insula.
A model proposed by Naqvi et al. (see above) is that the insula stores a representation of the pleasurable interoceptive effects of drug use (e.g., the airway sensory effects of nicotine, the cardiovascular effects of amphetamine), and that this representation is activated by exposure to cues that have previously been associated with drug use. A number of functional imaging studies have shown the insula to be activated during the administration of drugs of abuse. Several functional imaging studies have also shown that the insula is activated when drug users are exposed to drug cues, and that this activity is correlated with subjective urges. In the cue-exposure studies, insula activity is elicited when there is no actual change in the level of drug in the body. Therefore, rather than merely representing the interoceptive effects of drug use as it occurs, the insula may play a role in memory for the pleasurable interoceptive effects of past drug use, anticipation of these effects in the future, or both. Such a representation may give rise to conscious urges that feel as if they arise from within the body. This may make addicts feel as if their bodies need to use a drug, and may result in persons with lesions in the insula reporting that their bodies have forgotten the urge to use, according to this study.
Subjective certainty in ecstatic seizures
A common quality in mystical experiences is a strong feeling of certainty which cannot be expressed in words. Fabienne Picard proposes a neurological explanation for this subjective certainty, based on clinical research of epilepsy. [75] [76] According to Picard, this feeling of certainty may be caused by a dysfunction of the anterior insula, a part of the brain which is involved in interoception, self-reflection, and in avoiding uncertainty about the internal representations of the world by "anticipation of resolution of uncertainty or risk". This avoidance of uncertainty functions through the comparison between predicted states and actual states, that is, "signaling that we do not understand, i.e., that there is ambiguity."[77] Picard notes that "the concept of insight is very close to that of certainty," and refers to Archimedes "Eureka!"[78][79] Picard hypothesizes that in ecstatic seizures the comparison between predicted states and actual states no longer functions, and that mismatches between predicted state and actual state are no longer processed, blocking "negative emotions and negative arousal arising from predictive uncertainty," which will be experienced as emotional confidence.[80] Picard concludes that "[t]his could lead to a spiritual interpretation in some individuals."[80]
Other clinical conditions
The insular cortex has been suggested to have a role in anxiety disorders,[81] emotion dysregulation,[82] and anorexia nervosa.[83]
History
The insula was first described by Johann Christian Reil while describing cranial and spinal nerves and plexi.[84] Henry Gray in Gray's Anatomy is responsible for it being known as the Island of Reil.[84]
Additional images
 The insula of the left side, exposed by removing the opercula.
The insula of the left side, exposed by removing the opercula. Coronal section of brain through intermediate mass of third ventricle.
Coronal section of brain through intermediate mass of third ventricle. Coronal section through anterior cornua of lateral ventricles.
Coronal section through anterior cornua of lateral ventricles. Coronal section of brain through anterior commissure.
Coronal section of brain through anterior commissure.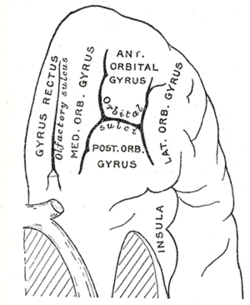 Orbital surface of left frontal lobe.
Orbital surface of left frontal lobe. Section of brain showing upper surface of temporal lobe.
Section of brain showing upper surface of temporal lobe. Horizontal section of left cerebral hemisphere.
Horizontal section of left cerebral hemisphere.- Human brain frontal (coronal) section
- Human brain view on transverse temporal and insular gyri
See also
References
- ↑ MUFSON, E; MESULAM, M; PANDYA, D (1 July 1981). "Insular interconnections with the amygdala in the rhesus monkey" (PDF). Neuroscience. 6 (7): 1231–1248. doi:10.1016/0306-4522(81)90184-6. PMID 6167896.
- ↑ Craig AD, Chen K, Bandy D, Reiman EM (2000). "Thermosensory activation of insular cortex". Nat. Neurosci. 3 (2): 184–90. doi:10.1038/72131. PMID 10649575.
- ↑ JAKAB, A; MOLNAR, P; BOGNER,P; BERES,M; BERENYI, E (1 Oct 2011). "Connectivity-based parcellation reveals interhemispheric differences in the insula". Brain Topography. 25 (3): 264–271. doi:10.1007/s10548-011-0205-y. PMID 22002490.
- 1 2 3 Johannes Sobotta. "Sobotta's Atlas and Text-book of human anatomy 1909". Sobotta's Atlas and Text-book of human anatomy 1909. p. 145. Retrieved November 10, 2013.
- ↑ "Definition: 'Circular Sulcus Of Insula'". MediLexicon. Retrieved 2012-03-30.
- ↑ Bauernfeind A; et al. (April 2013). "A volumetric comparison of the insular cortex and its subregions in primates". Human Evolution. 64 (4): 263–279. doi:10.1016/j.hevol.2012.12.003 (inactive 2016-06-29).
- ↑ Brain, MSN Encarta. Archived 2009-10-31.
- ↑ Kolb, Bryan; Whishaw, Ian Q. (2003). Fundamentals of human neuropsychology (5th ed.). [New York]: Worth. ISBN 0-7167-5300-6.
- ↑ Bushara, Khalaf e t al (2001 Jan 1). "Neural correlates of auditory-visual stimulus onset asynchrony detection.". J Neurosci. 21 (1): 300–4. PMID 11150347. Check date values in:
|date=(help) - ↑ Bushara, Khalaf; et al. (2003 Feb). "Neural correlates of cross-modal binding.". Nat Neurosci. 6 (2): 190–5. PMID 12496761. Check date values in:
|date=(help) - ↑ Benedetto De Martino; Dharshan Kumaran; Ben Seymour; Raymond J. Dolan (August 2006). "Frames, Biases, and Rational Decision-Making in the Human Brain". Science. 313 (6): 684–687. Bibcode:2006Sci...313..684D. doi:10.1126/science.1128356. PMC 2631940
 . PMID 16888142.
. PMID 16888142. - ↑ Gui Xue; Zhonglin Lu; Irwin P. Levin d; Antoine Bechara (2010). "The impact of prior risk experiences on subsequent risky decision-making: The role of the insula". NeuroImage. 50 (2): 709–716. doi:10.1016/j.neuroimage.2009.12.097. PMC 2828040
 . PMID 20045470.
. PMID 20045470. - ↑ Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ (February 2004). "Neural systems supporting interoceptive awareness". Nat. Neurosci. 7 (2): 189–95. doi:10.1038/nn1176. PMID 14730305.
- 1 2 Lamb K, Gallagher K, McColl R, Mathews D, Querry R, Williamson JW (April 2007). "Exercise-induced decrease in insular cortex rCBF during postexercise hypotension". Med Sci Sports Exerc. 39 (4): 672–9. doi:10.1249/mss.0b013e31802f04e0. PMID 17414805.
- ↑ Williamson JW, McColl R, Mathews D, Mitchell JH, Raven PB, Morgan WP (April 2001). "Hypnotic manipulation of effort sense during dynamic exercise: cardiovascular responses and brain activation". J. Appl. Physiol. 90 (4): 1392–9. PMID 11247939.
- ↑ Williamson JW, McColl R, Mathews D, Ginsburg M, Mitchell JH (September 1999). "Activation of the insular cortex is affected by the intensity of exercise". J. Appl. Physiol. 87 (3): 1213–9. PMID 10484598.
- ↑ Baliki MN, Geha PY, Apkarian AV (February 2009). "Parsing pain perception between nociceptive representation and magnitude estimation". J. Neurophysiol. 101 (2): 875–87. doi:10.1152/jn.91100.2008. PMC 3815214
 . PMID 19073802.
. PMID 19073802. - ↑ Ogino Y, Nemoto H, Inui K, Saito S, Kakigi R, Goto F (May 2007). "Inner experience of pain: imagination of pain while viewing images showing painful events forms subjective pain representation in human brain". Cereb. Cortex. 17 (5): 1139–46. doi:10.1093/cercor/bhl023. PMID 16855007.
- ↑ Song GH, Venkatraman V, Ho KY, Chee MW, Yeoh KG, Wilder-Smith CH (December 2006). "Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls". Pain. 126 (1–3): 79–90. doi:10.1016/j.pain.2006.06.017. PMID 16846694.
- ↑ Olausson H, Charron J, Marchand S, Villemure C, Strigo IA, Bushnell MC (November 2005). "Feelings of warmth correlate with neural activity in right anterior insular cortex". Neurosci. Lett. 389 (1): 1–5. doi:10.1016/j.neulet.2005.06.065. PMID 16051437.
- ↑ Craig AD, Chen K, Bandy D, Reiman EM (February 2000). "Thermosensory activation of insular cortex". Nat. Neurosci. 3 (2): 184–90. doi:10.1038/72131. PMID 10649575.
- ↑ Ladabaum U, Minoshima S, Hasler WL, Cross D, Chey WD, Owyang C (February 2001). "Gastric distention correlates with activation of multiple cortical and subcortical regions". Gastroenterology. 120 (2): 369–76. doi:10.1053/gast.2001.21201. PMID 11159877.
- ↑ Hamaguchi T, Kano M, Rikimaru H, et al. (June 2004). "Brain activity during distention of the descending colon in humans". Neurogastroenterol. Motil. 16 (3): 299–309. doi:10.1111/j.1365-2982.2004.00498.x. PMID 15198652.
- ↑ Matsuura S, Kakizaki H, Mitsui T, Shiga T, Tamaki N, Koyanagi T (November 2002). "Human brain region response to distention or cold stimulation of the bladder: a positron emission tomography study". J. Urol. 168 (5): 2035–9. doi:10.1097/01.ju.0000027600.26331.11 (inactive 2016-06-29). PMID 12394703.
- ↑ von Leupoldt, A.; Sommer, T.; Kegat, S.; Baumann, H. J.; Klose, H.; Dahme, B.; Buchel, C. (24 January 2008). "The Unpleasantness of Perceived Dyspnea Is Processed in the Anterior Insula and Amygdala" (PDF). American Journal of Respiratory and Critical Care Medicine. 177 (9): 1026–1032. doi:10.1164/rccm.200712-1821OC. PMID 18263796.
- ↑ Kikuchi M, Naito Y, Senda M, et al. (April 2009). "Cortical activation during optokinetic stimulation — an fMRI study". Acta Otolaryngol. 129 (4): 440–3. doi:10.1080/00016480802610226. PMID 19116795.
- ↑ Papathanasiou ES, Papacostas SS, Charalambous M, Eracleous E, Thodi C, Pantzaris M (2006). "Vertigo and imbalance caused by a small lesion in the anterior insula". Electromyogr Clin Neurophysiol. 46 (3): 185–92. PMID 16918202.
- ↑ Brown S, Martinez MJ, Parsons LM (September 2004). "Passive music listening spontaneously engages limbic and paralimbic systems". NeuroReport. 15 (13): 2033–7. doi:10.1097/00001756-200409150-00008. PMID 15486477.
- ↑ Sander K, Scheich H (October 2005). "Left auditory cortex and amygdala, but right insula dominance for human laughing and crying". J Cogn Neurosci. 17 (10): 1519–31. doi:10.1162/089892905774597227. PMID 16269094.
- ↑ http://ccare.stanford.edu/node/89
- ↑ Bamiou DE, Musiek FE, Luxon LM (May 2003). "The insula (Island of Reil) and its role in auditory processing. Literature review". Brain Res. Brain Res. Rev. 42 (2): 143–54. doi:10.1016/S0165-0173(03)00172-3. PMID 12738055.
- ↑ Anderson TJ, Jenkins IH, Brooks DJ, Hawken MB, Frackowiak RS, Kennard C (October 1994). "Cortical control of saccades and fixation in man. A PET study". Brain. 117 (Pt 5): 1073–84. doi:10.1093/brain/117.5.1073. PMID 7953589.
- ↑ Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE (April 1997). "Multiple nonprimary motor areas in the human cortex". J. Neurophysiol. 77 (4): 2164–74. PMID 9114263.
- ↑ Sörös P, Inamoto Y, Martin RE (August 2009). "Functional brain imaging of swallowing: an activation likelihood estimation meta-analysis". Hum Brain Mapp. 30 (8): 2426–39. doi:10.1002/hbm.20680. PMID 19107749.
- ↑ Penfield W, Faulk ME (1955). "The insula; further observations on its function". Brain. 78 (4): 445–70. doi:10.1093/brain/78.4.445. PMID 13293263.
- ↑ Dronkers NF (November 1996). "A new brain region for coordinating speech articulation". Nature. 384 (6605): 159–61. Bibcode:1996Natur.384..159D. doi:10.1038/384159a0. PMID 8906789.
- ↑ Ackermann H, Riecker A (May 2004). "The contribution of the insula to motor aspects of speech production: a review and a hypothesis". Brain Lang. 89 (2): 320–8. doi:10.1016/S0093-934X(03)00347-X. PMID 15068914.
- ↑ Nowak M, Holm S, Biering-Sørensen F, Secher NH, Friberg L (June 2005). ""Central command" and insular activation during attempted foot lifting in paraplegic humans". Hum Brain Mapp. 25 (2): 259–65. doi:10.1002/hbm.20097. PMID 15849712.
- ↑ Borovsky A, Saygin AP, Bates E, Dronkers N (June 2007). "Lesion correlates of conversational speech production deficits". Neuropsychologia. 45 (11): 2525–33. doi:10.1016/j.neuropsychologia.2007.03.023. PMID 17499317.
- ↑ Mutschler I, Schulze-Bonhage A, Glauche V, Demandt E, Speck O, Ball T (2007). Fitch T, ed. "A rapid sound-action association effect in human insular cortex". PLoS ONE. 2 (2): e259. Bibcode:2007PLoSO...2..259M. doi:10.1371/journal.pone.0000259. PMC 1800344
 . PMID 17327919.
. PMID 17327919. - ↑ Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS (February 1993). "Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction". Annals of Neurology. 33 (2): 181–9. doi:10.1002/ana.410330208. PMID 8434880.
- ↑ Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC (September 1992). "Cardiovascular effects of human insular cortex stimulation". Neurology. 42 (9): 1727–32. doi:10.1212/wnl.42.9.1727. PMID 1513461.
- ↑ Critchley HD (December 2005). "Neural mechanisms of autonomic, affective, and cognitive integration". J. Comp. Neurol. 493 (1): 154–66. doi:10.1002/cne.20749. PMID 16254997.
- ↑ Pacheco-López G, Niemi MB, Kou W, Härting M, Fandrey J, Schedlowski M (March 2005). "Neural substrates for behaviorally conditioned immunosuppression in the rat". J. Neurosci. 25 (9): 2330–7. doi:10.1523/JNEUROSCI.4230-04.2005. PMID 15745959.
- ↑ Ramírez-Amaya V, Alvarez-Borda B, Ormsby CE, Martínez RD, Pérez-Montfort R, Bermúdez-Rattoni F (June 1996). "Insular cortex lesions impair the acquisition of conditioned immunosuppression". Brain Behav. Immun. 10 (2): 103–14. doi:10.1006/brbi.1996.0011. PMID 8811934.
- ↑ Ramírez-Amaya V, Bermúdez-Rattoni F (March 1999). "Conditioned enhancement of antibody production is disrupted by insular cortex and amygdala but not hippocampal lesions". Brain Behav. Immun. 13 (1): 46–60. doi:10.1006/brbi.1998.0547. PMID 10371677.
- ↑ Karnath HO, Baier B, Nägele T (August 2005). "Awareness of the functioning of one's own limbs mediated by the insular cortex?". J. Neurosci. 25 (31): 7134–8. doi:10.1523/JNEUROSCI.1590-05.2005. PMID 16079395.
- ↑ Craig AD (January 2009). "How do you feel—now? The anterior insula and human awareness". Nature Reviews Neuroscience. 10 (1): 59–70. doi:10.1038/nrn2555. PMID 19096369.
- ↑ Farrer C, Frith CD (March 2002). "Experiencing oneself vs another person as being the cause of an action: the neural correlates of the experience of agency". NeuroImage. 15 (3): 596–603. doi:10.1006/nimg.2001.1009. PMID 11848702.
- ↑ Tsakiris M, Hesse MD, Boy C, Haggard P, Fink GR (October 2007). "Neural signatures of body ownership: a sensory network for bodily self-consciousness". Cereb. Cortex. 17 (10): 2235–44. doi:10.1093/cercor/bhl131. PMID 17138596.
- ↑ Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G (October 2003). "Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust". Neuron. 40 (3): 655–64. doi:10.1016/S0896-6273(03)00679-2. PMID 14642287.
- ↑ Wright P, He G, Shapira NA, Goodman WK, Liu Y (October 2004). "Disgust and the insula: fMRI responses to pictures of mutilation and contamination". NeuroReport. 15 (15): 2347–51. doi:10.1097/00001756-200410250-00009. PMID 15640753.
- ↑ Jabbi M, Bastiaansen J, Keysers C (2008). Lauwereyns J, ed. "A common anterior insula representation of disgust observation, experience and imagination shows divergent functional connectivity pathways". PLoS ONE. 3 (8): e2939. Bibcode:2008PLoSO...3.2939J. doi:10.1371/journal.pone.0002939. PMC 2491556
 . PMID 18698355.
. PMID 18698355. - ↑ Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD (June 2003). "The neural basis of economic decision-making in the Ultimatum Game". Science. 300 (5626): 1755–8. Bibcode:2003Sci...300.1755S. doi:10.1126/science.1082976. PMID 12805551.
- ↑ Phan KL, Wager T, Taylor SF, Liberzon I (June 2002). "Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI". NeuroImage. 16 (2): 331–48. doi:10.1006/nimg.2002.1087. PMID 12030820.
- ↑ Singer T (2006). "The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research". Neurosci Biobehav Rev. 30 (6): 855–63. doi:10.1016/j.neubiorev.2006.06.011. PMID 16904182.
- ↑ Ortigue S, Grafton ST, Bianchi-Demicheli F (August 2007). "Correlation between insula activation and self-reported quality of orgasm in women". NeuroImage. 37 (2): 551–60. doi:10.1016/j.neuroimage.2007.05.026. PMID 17601749.
- ↑ Quarto, Tiziana; Blasi, Giuseppe; Maddalena, Chiara; Viscanti, Giovanna; Lanciano, Tiziana; Soleti, Emanuela; Mangiulli, Ivan; Taurisano, Paolo; Fazio, Leonardo (2016-02-09). "Association between Ability Emotional Intelligence and Left Insula during Social Judgment of Facial Emotions". PLOS ONE. 11 (2): e0148621. doi:10.1371/journal.pone.0148621. ISSN 1932-6203. PMC 4747486
 . PMID 26859495.
. PMID 26859495. - ↑ Wager, Tor (June 2002). "Functional Neuroanatomy of Emotion: A Meta-Analysis of Emotion Activation Studies in PET and fMRI". NeuroImage. 16 (2): 331–48. doi:10.1006/nimg.2002.1087. PMID 12030820.
- ↑ Vilares I, Howard JD, Fernandes HL, Gottfried JA, Kording KP (2012). "Differential Representations of Prior and Likelihood Uncertainty in the Human Brain". Current Biology. 22 (18): 1641–1648. doi:10.1016/j.cub.2012.07.010. PMC 3461114
 . PMID 22840519.
. PMID 22840519. - ↑ Craig, A. D. (Bud) (2009). "How do you feel — now? The anterior insula and human awareness" (PDF). Nature Reviews Neuroscience. 10 (1): 59–70. doi:10.1038/nrn2555. PMID 19096369.
- ↑ Craig, A. D. (Bud) (2002). "A new view of pain as a homeostatic emotion" (PDF). Trends in Neuroscience. 26 (6): 303–307. doi:10.1016/s0166-2236(03)00123-1.
- ↑ Sara W. Lazar; Catherine E. Kerr; Rachel H. Wasserman; Jeremy R. Gray; Douglas N. Greve; Michael T. Treadway; Metta McGarvey; Brian T. Quinn; Jeffery A. Dusek; Herbert Benson; Scott L. Rauch; Christopher I. Moore; Bruce Fischl (2005). "Meditation experience is associated with increased cortical thickness". NeuroReport. 16 (17): 1893–7. doi:10.1097/01.wnr.0000186598.66243.19. PMC 1361002
 . PMID 16272874.
. PMID 16272874. - ↑ http://scan.oxfordjournals.org/cgi/content/full/3/1/55
- ↑ Taylor KS, Seminowicz DA, Davis KD (September 2009). "Two systems of resting state connectivity between the insula and cingulate cortex". Hum Brain Mapp. 30 (9): 2731–45. doi:10.1002/hbm.20705. PMID 19072897.
- 1 2 Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR (2009). "At the heart of the ventral attention system: the right anterior insula". Hum Brain Mapp. 30 (8): 2530–41. doi:10.1002/hbm.20688. PMC 2712290
 . PMID 19072895.
. PMID 19072895. - ↑ Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR (November 2003). "Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula". Brain. 126 (Pt 11): 2406–18. doi:10.1093/brain/awg240. PMID 12902311.
- ↑ Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. (March 2004). "Cognition and anatomy in three variants of primary progressive aphasia". Annals of Neurology. 55 (3): 335–46. doi:10.1002/ana.10825. PMC 2362399
 . PMID 14991811.
. PMID 14991811. - ↑ Nasir H. Naqvi; David Rudrauf; Hanna Damasio; Antoine Bechara. (January 2007). "Damage to the Insula Disrupts Addiction to Cigarette Smoking" (abstract). Science. 315 (5811): 531–4. Bibcode:2007Sci...315..531N. doi:10.1126/science.1135926. PMC 3698854
 . PMID 17255515.
. PMID 17255515. - ↑ Vorel SR, Bisaga A, McKhann G, Kleber HD (July 2007). "Insula damage and quitting smoking". Science. 317 (5836): 318–9; author reply 318–9. doi:10.1126/science.317.5836.318c. PMID 17641181.
- ↑ Suner-Soler, R. (2011). "Smoking Cessation 1 Year Poststroke and Damage to the Insular Cortex". Stroke. 43 (1): 131–136. doi:10.1161/STROKEAHA.111.630004. PMID 22052507.
- ↑ Gaznick, N. (2013). "Basal Ganglia Plus Insula Damage Yields Stronger Disruption of Smoking Addiction Than Basal Ganglia Damage Alone". Nicotine. 16 (4): 445–453. doi:10.1093/ntr/ntt172.
- ↑ Hyman, Steven E. (2005-08-01). "Addiction: A Disease of Learning and Memory" (abstract). Am J Psychiatry. 162 (8): 1414–22. doi:10.1176/appi.ajp.162.8.1414. PMID 16055762.
- ↑ Marco Contreras; Francisco Ceric; Fernando Torrealba (January 2007). "Inactivation of the Interoceptive Insula Disrupts Drug Craving and Malaise Induced by Lithium" (abstract). Science. 318 (5850): 655–8. Bibcode:2007Sci...318..655C. doi:10.1126/science.1145590. PMID 17962567.
- ↑ Picard, Fabienne (2013), "State of belief, subjective certainty and bliss as a product of cortical dysfuntion", Cortex, 49 (9): 2494–2500, doi:10.1016/j.cortex.2013.01.006, PMID 23415878
- ↑ Gschwind, Markus; Picard, Fabienne (2016), "Ecstatic Epileptic Seizures: a glimpse into the multiple roles of the insula", Frontiers in Behavioral Neuroscience, 10, doi:10.3389/fnbeh.2016.00021
- ↑ Picard 2013, p.2496-2498
- ↑ Picard 2013, p.2497-2498
- ↑ See also satori in Japanese Zen
- 1 2 Picard 2013, p.2498
- ↑ Paulus MP, Stein MB (August 2006). "An insular view of anxiety". Biol. Psychiatry. 60 (4): 383–7. doi:10.1016/j.biopsych.2006.03.042. PMID 16780813.
- ↑ Thayer JF, Lane RD (December 2000). "A model of neurovisceral integration in emotion regulation and dysregulation". J Affect Disord. 61 (3): 201–16. doi:10.1016/S0165-0327(00)00338-4. PMID 11163422.
- ↑ Gaudio S, Wiemerslage L, Brooks SJ, Schiöth HB (2016). "A systematic review of resting-state functional-MRI studies in anorexia nervosa: Evidence for functional connectivity impairment in cognitive control and visuospatial and body-signal integration.". Neurosci Biobehav Rev. doi:10.1016/j.neubiorev.2016.09.032. PMID 27725172.
- 1 2 Binder DK, Schaller K, Clusmann H (November 2007). "The seminal contributions of Johann-Christian Reil to anatomy, physiology, and psychiatry". Neurosurgery. 61 (5): 1091–6; discussion 1096. doi:10.1227/01.neu.0000303205.15489.23. PMID 18091285.
External links
| Wikimedia Commons has media related to Insular cortex. |
- Insular cortex in the Brede Database at the Technical University of Denmark. Location and literature citations for the insula
- synd/1212 at Who Named It?
- Anatomy diagram: 13048.000-1 at Roche Lexicon - illustrated navigator, Elsevier
- Stained brain slice images which include the "insular cortex" at the BrainMaps project
- Thomas P. Naidicha; et al. (1 February 2004). "The Insula: Anatomic Study and MR Imaging Display at 1.5 T". American Journal of Neuroradiology. 25 (2): 222–32. PMID 14970021.
- Kakigia R, Nakataa H, Inuia K, Hiroea N, et al. (October 2005). "Intracerebral pain processing in a Yoga Master who claims not to feel pain during meditation". Eur J Pain. 9 (5): 581–9. doi:10.1016/j.ejpain.2004.12.006. PMID 16139187.
As for fMRI recording, there were remarkable changes in levels of activity in the...SII-insula (mainly the insula)