Hydralazine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Apresoline |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682246 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth, intravenous |
| ATC code | C02DB02 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 26–50% |
| Protein binding | 85–90% |
| Metabolism | Liver |
| Biological half-life | 2–8 hours, 7–16 hours (renal impairment) |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number |
86-54-4 |
| PubChem (CID) | 3637 |
| IUPHAR/BPS | 7326 |
| DrugBank |
DB01275 |
| ChemSpider |
3511 |
| UNII |
26NAK24LS8 |
| KEGG |
D08044 |
| ChEBI |
CHEBI:5775 |
| ChEMBL |
CHEMBL276832 |
| ECHA InfoCard | 100.001.528 |
| Chemical and physical data | |
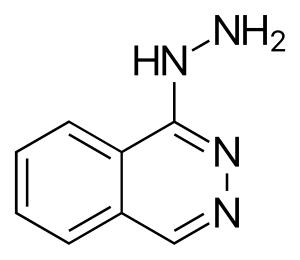
| Formula | C8H8N4 |
| Molar mass | 160.176 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Hydralazine (trade name Apresoline) is a direct-acting smooth muscle relaxant used to treat hypertension by acting as a vasodilator primarily in arteries and arterioles. By relaxing vascular smooth muscle, vasodilators act to decrease peripheral resistance, thereby lowering blood pressure and decreasing afterload.[1]
However, this only has a short term effect on blood pressure, as the system will reset to the previous, high blood pressure needed to maintain pressure in the kidney necessary for natriuresis. The long-term effect of antihypertensive drugs comes from their effects on the pressure natriuresis curve. It belongs to the hydrazinophthalazine class of drugs.[2]
It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[3]
Medical use
Hydralazine is not used as a primary drug for treating hypertension because it elicits a reflex sympathetic stimulation of the heart (the baroreceptor reflex).[4] The sympathetic stimulation may increase heart rate and cardiac output, and in patients with coronary artery disease may cause angina pectoris or myocardial infarction.[1] Hydralazine may also increase plasma renin concentration, resulting in fluid retention. To prevent these undesirable side effects, hydralazine is usually prescribed in combination with a β-blocker (e.g., propranolol) and a diuretic.[1] In the UK, labetalol tends to be the first-line β-blocker.
Hydralazine is used to treat severe hypertension, but again, it is not a first-line therapy for essential hypertension. However, hydralazine is the first-line therapy for hypertension in pregnancy, with methyldopa.[5] It has also been used successfully as a treatment for myelodysplastic syndrome in its capacity as a DNA methyltransferase inhibitor.[6]
Hydralazine is commonly used in combination with isosorbide dinitrate for the treatment of congestive heart failure in self-identified African American populations. This preparation, BiDil, was the first race-based prescription drug.
Side effects
Very common (>10% frequency) side effects include:[7]
Common (1–10% frequency) side effects include:[7][8]
- Flushing
- Hypotension
- Anginal symptoms
- Joint ache
- Positive test for ANP
- Gastrointestinal disturbances
- Diarrhea
- Nausea
- Vomiting
- Joint swelling
- Muscle aches
- Edema (sodium and water retention)
Uncommon (0.1–1% frequency) side effects include:[7][8]
- Nasal congestion
- Heart failure
- Dizziness
- Rash
- Lupus-like syndrome
- Protein in the urine
- Increased plasma creatinine
- Blood in the urine
- Glomerulonephritis
- Jaundice
- Liver enlargement
- Hepatitis
- Agitation
- Weight loss
- Appetite loss
- Anxiety
- Blood dyscrasias
- Increased lacrimation
- Conjunctivitis
- Shortness of breath
- Pleural pain
- Fever
- Malaise
- Hypersensitivity reactions
Rare (<0.1% frequency) side effects include:[7][8]
- Paradoxical pressor responses
- Pins and needles (might be reversed by pyridoxine administration)
- Peripheral neuritis
- Polyneuritis
- Tremor
- Paralyzed bowel
- Acute kidney failure
- Urinary retention
- Depression
- Hallucinations
- Haemolytic anaemia
- Leukocytosis
- Lymphadenopathy
- Pancytopenia
- Splenomegaly
- Agranulocytosis
- Exophthalmos
- Retroperitoneal fibrosis
Contraindications
Contraindications include:[7]
- Known hypersensitivity to hydralazine or dihydralazine
- Idiopathic systemic lupus erythematosus and related diseases
- Severe tachycardia and heart failure with a high cardiac output (e.g. in thyrotoxicosis)
- Myocardial insufficiency due to mechanical obstruction (e.g. in the presence of aortic or mitral stenosis or constrictive pericarditis)
- Isolated right-ventricular heart failure due to pulmonary hypertension (cor pulmonale)
- Dissecting aortic aneurysm
Interactions
It may potentiate the antihypertensive effects of:[7]
Drugs subject to a strong first-pass effect such as β-blockers may increase the bioavailability of hydralazine.[7] Epinephrine (adrenaline)'s heart rate-accelerating effects are increased by hydralazine, hence may lead to toxicity.[7]
Mechanism of action
Hydralazine causes arterial vasodilation by an, as of yet, unclarified mechanism. Hydralazine requires the endothelium to provide nitric oxide,[9] thus only causes vasodilation in vivo with functional endothelium. Hydralazine will not cause vasodilation in vitro in an isolated blood vessel.
Activation of hypoxia-inducible factors has been suggested as a mechanism.[10]
See also
References
- 1 2 3 Harvey, Richard A., Pamela A. Harvey, and Mark J. Mycek. Lippincott's Illustrated Reviews: Pharmacology. 2nd ed. Philadelphia: Lipincott, Williams & Wilkins, 2000. 190.
- ↑ Bourreli, B.; Pinaud, M.; Passuti, N.; Gunst, J. P.; Drouet, J. C.; Remi, J. P. (1988). "Additive effects of dihydralazine during enflurane or isoflurane hypotensive anaesthesia for spinal fusion". Canadian Journal of Anesthesia. 35 (3): 242–8. doi:10.1007/BF03010617. PMID 3383316.
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ Kandler MR, Mah GT, Tejani AM, Stabler SN, Salzwedel DM. Hydralazine for essential hypertension. Cochrane Database of Systematic Reviews 2011, Issue 11. Art. No.: CD004934. DOI: 10.1002/14651858.CD004934.pub4.
- ↑ Bhushan, Vikas, Tao T. Lee, and Ali Ozturk. First Aid for the USMLE Step 1. New York: McGraw-Hill Medical, 2007. 251.
- ↑ Candelaria, M; Herrera, A; Labardini, J; González-Fierro, A; Trejo-Becerril, C; Taja-Chayeb, L; Pérez-Cárdenas, E; Cruz-Hernández, E; Arias-Bofill, D; Vidal, S; Cervera, E; Dueñas-Gonzalez, A (5 October 2010). "Hydralazine and magnesium valproate as epigenetic treatment for myelodysplastic syndrome. Preliminary results of a phase-II trial". Annals of Hematology. 90 (4): 379–387. doi:10.1007/s00277-010-1090-2. PMID 20922525.
- 1 2 3 4 5 6 7 8 "PRODUCT INFORMATION APRESOLINE® (hydralazine hydrochloride 20 mg powder for injection ampoule)" (PDF). TGA eBusiness Services. Link Medical Products Pty Ltd. 27 March 2005. Retrieved 13 February 2014.
- 1 2 3 Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ↑ "antihtn". Retrieved 2008-10-05.
- ↑ Knowles HJ, Tian YM, Mole DR, Harris AL (July 2004). "Novel mechanism of action for hydralazine: induction of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by inhibition of prolyl hydroxylases". Circ. Res. 95 (2): 162–9. doi:10.1161/01.RES.0000134924.89412.70. PMID 15192023.