Hookworm vaccine
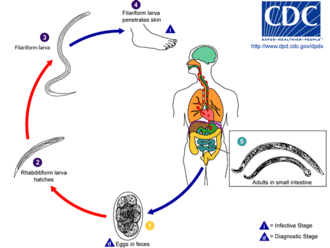
Hookworm vaccine is a vaccine against hookworm.[1] No effective vaccine for the disease has yet been developed. Hookworms, parasitic nematodes transmitted in soil, infect approximately 700 million humans, particularly in tropical regions of the world where endemic hookworms include Ancylostoma duodenale and Necator americanus. Hookworms feed on blood and those infected with hookworms may suffer from chronic anaemia and malnutrition.[1][2] Helminth infection can be effectively treated with benzimidazole drugs (such as mebendazole or albendazole), and efforts led by the World Health Organization have focused on one to three yearly de-worming doses in schools because hookworm infections with the heaviest intensities are most common in school-age children.[3] However, these drugs only eliminate existing adult parasites and re-infection can occur soon after treatment, school-based de-worming efforts do not treat adults or pre-school children, and concerns exist about drug resistance developing in hookworms against the commonly used treatments, thus a vaccine against hookworm disease is sought to provide more permanent resistance to infection.[3][4]
Mechanism of action
Hookworm infections in humans can last for several years, and re-infection can occur very shortly after treatment, suggesting that hookworms effectively evade—and may interrupt or modulate—the host immune system.[1] Successful hookworm vaccines have been developed for several animal species.[1] On the basis of prior work, human vaccine development has targeted antigens from both the larval and adult stages of the hookworm life cycle; a combined vaccine for humans that would provide more complete protection.[1] Current targets of larval proteins attenuate larval migration through host tissue; targets of adult proteins have been demonstrated to block enzymes vital to hookworm feeding.
Examples of antigenic targets of hookworm vaccines currently in clinical trials include Na-ASP-2 from N. americanus, Ac-APR-1 from Ancylostoma caninum, Ac'-APR-1 and Na-GST-1/Alhydroge.[5]
The function of Na-ASP-2 is not currently known (though it may function as a chemotaxin mimic[1]), but it is a cysteine-rich secretory protein that is released during parasite entry into the host and may have some function in the transition from the larval environment stage of the hookworm life-cycle to an adult parasitic existence.[2][6][7][8] The class of proteins that includes Na-ASP-2, ASPs, are promising vaccine candidates based on previous vaccine studies in sheep, guinea pigs, cattle, and mice, which have demonstrated inhibition of hookworm larval migration. Furthermore, epidemiologic studies determined that high titers of circulating antibodies against ASPs are associated with lower hookworm burdens in residents of Hainan Province, China, and Minas Gerais, Brazil.[9]
Ac-APR-1 is an aspartic protease, specifically a hemoglobinase, that participates in the hookworm's digestion of hemoglobin from its blood meal[4] and is present in the adult stage of the hookworm life cycle.[1][4] Animals immunized against Ac-APR-1 exhibited a reduction in worm burden, a reduction in hemoglobin loss, and a dramatic reduction in worm fecundity.[2]
In 2014 Na-GST-1/Alhydroge completed a successful phase 1 clinical trial in Brazil.[5]
Research support
Hookworm infection has been considered a "neglected disease" that disproportionately affects poorer localities and has received little attention from pharmaceutical companies.[10] Support for current research efforts to develop hookworm vaccines has come from the Human Hookworm Vaccine Initiative,[11] a program of the Sabin Vaccine Institute and collaborations with George Washington University, the Oswaldo Cruz Foundation, the Chinese Institute of Parasitic Diseases, the Queensland Institute of Medical Research, and the London School of Hygiene and Tropical Medicine.[2][12][13] Funding for hookworm vaccine research efforts includes substantial money from the Bill & Melinda Gates Foundation,[14][15] totaling in excess of $53 million,[10] and additional support from the Rockefeller Foundation, Doctors Without Borders, National Institute of Allergy and Infectious Diseases, and the March of Dimes Birth Defects Foundation.[10][13]
The government of Brazil, where hookworm is still endemic in some poorer areas, has promised to manufacture a vaccine if one can be proven effective.[16]
References
- 1 2 3 4 5 6 7 Diemert DJ, Bethony JM, Hotez PJ (January 2008). "Hookworm vaccines". Clin. Infect. Dis. 46 (2): 282–8. doi:10.1086/524070. PMID 18171264.
- 1 2 3 4 Devaney E (October 2005). "The End of the Line for Hookworm? An Update on Vaccine Development". PLoS Med. 2 (10): e327. doi:10.1371/journal.pmed.0020327. PMC 1240053
 . PMID 16187734. View on Wikipedia
. PMID 16187734. View on Wikipedia - 1 2 Hotez PJ, Bethony J, Bottazzi ME, Brooker S, Buss P (March 2005). "Hookworm: "The Great Infection of Mankind"". PLoS Med. 2 (3): e67. doi:10.1371/journal.pmed.0020067. PMC 1069663
 . PMID 15783256.
. PMID 15783256. - 1 2 3 Loukas A, Bethony JM, Mendez S, et al. (October 2005). "Vaccination with Recombinant Aspartic Hemoglobinase Reduces Parasite Load and Blood Loss after Hookworm Infection in Dogs". PLoS Med. 2 (10): e295. doi:10.1371/journal.pmed.0020295. PMC 1240050
 . PMID 16231975.
. PMID 16231975. - 1 2 "Phase 1 Clinical Trial of Human Hookworm Vaccine Successfully Completed | Sabin". www.sabin.org. Retrieved 2016-06-11.
- ↑ Fujiwara RT, Bethony J, Bueno LL, et al. (2005). "Immunogenicity of the hookworm Na-ASP-2 vaccine candidate: characterization of humoral and cellular responses after vaccination in the Sprague Dawley rat". Hum Vaccin. 1 (3): 123–8. doi:10.4161/hv.1.3.1924. PMID 17012856.
- ↑ Bethony JM, Simon G, Diemert DJ, et al. (May 2008). "Randomized, placebo-controlled, double-blind trial of the Na-ASP-2 hookworm vaccine in unexposed adults". Vaccine. 26 (19): 2408–17. doi:10.1016/j.vaccine.2008.02.049. PMID 18396361.
- ↑ Goud GN, Bottazzi ME, Zhan B, et al. (September 2005). "Expression of the Necator americanus hookworm larval antigen Na-ASP-2 in Pichia pastoris and purification of the recombinant protein for use in human clinical trials". Vaccine. 23 (39): 4754–64. doi:10.1016/j.vaccine.2005.04.040. PMID 16054275.
- ↑ Hotez PJ, Zhan B, Bethony JM, et al. (September 2003). "Progress in the development of a recombinant vaccine for human hookworm disease: the Human Hookworm Vaccine Initiative". Int. J. Parasitol. 33 (11): 1245–58. doi:10.1016/S0020-7519(03)00158-9. PMID 13678639.
- 1 2 3 In Brazil, Field Trials To Treat World's Poor, Washington Post, October 11, 2006
- ↑ Bottazzi ME, Brown AS (December 2008). "Model for product development of vaccines against neglected tropical diseases: a vaccine against human hookworm". Expert Rev Vaccines. 7 (10): 1481–92. doi:10.1586/14760584.7.10.1481. PMID 19053205.
- ↑ Human Hookworm Vaccine Initiative Overview, Sabin Vaccine Institute
- 1 2 World Health Organization, Initiative for Vaccine Research, Hookworm disease
- ↑ Researchers Cite Vaccines' Potential to Break Poverty Cycle; Genomics and Innovative Partnerships are Key to Parasitic and Bacterial Vaccine Development, Infection Control Today
- ↑ Sabin Vaccine Institute Receives $13.8M Gates Foundation Grant to Pursue Bivalent Hookworm Vaccination Strategy, redOrbit.com
- ↑ "In Brazil, a New Effort to Wipe Out Hookworm". 2005-10-29. Retrieved 2011-08-28.
External links
- Study of Na-ASP-2 Human Hookworm Vaccine in Healthy Adults Without Evidence of Hookworm Infection, ClinicalTrials.gov
- Phase 1 Trial of Na-ASP-2 Hookworm Vaccine in Previously Infected Brazilian Adults, ClinicalTrials.gov