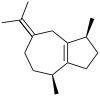
Guaiene
| |||
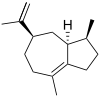 δ-Guaiene | |||
| Names | |||
|---|---|---|---|
| IUPAC names
α: (1S,4S,7R)-1,4-Dimethyl-7-(prop-1-en-2-yl)-1,2,3,4,5,6,7,8-octahydroazulene β: (1S,4S)-1,4-Dimethyl-7-(propan-2-ylidene)-1,2,3,4,5,6,7,8-octahydroazulene δ: (3S,3aS,5R)-3,8-Dimethyl-5-(prop-1-en-2-yl)-1,2,3,3a,4,5,6,7-octahydroazulene | |||
| Other names
Guajene | |||
| Identifiers | |||
| 3691-12-1 (α) 88-84-6 (β) 3691-11-0 (δ) | |||
| 3D model (Jmol) | (α): Interactive image (β): Interactive image (δ): Interactive image | ||
| ChemSpider | 4476575 (α) 16736689 (β) 85080 (δ) | ||
| ECHA InfoCard | 100.169.245 | ||
| PubChem | 5317844 (α) 15560252 (β) 94275 (δ) | ||
| UNII | 1D018Q907T (β) | ||
| |||
| |||
| Properties | |||
| C15H24 | |||
| Molar mass | 204.36 g·mol−1 | ||
| Boiling point | α: 281-282 °C[1] α: 78-79 °C (@ 2.5 Torr)[2] β: 281 °C[3] | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Guaienes are a series of closely related natural chemical compounds that have been isolated from a variety of plant sources. The guaienes are sesquiterpenes with the molecular formula C15H24. α-Guaiene is the most common and was first isolated from guaiac wood oil from Bulnesia sarmientoi.[4] The guaienes are used in the fragrance and flavoring industries to impart earthy, spicy aromas and tastes.[1][5]
See also
References
- 1 2 Alpha-guaiene, The Good Scents Company
- ↑ Takeda, Kenichi (1961). "Studies on sesquiterpenoids—III, Some derivatives of guaiol". Tetrahedron. 13 (4): 308–318. doi:10.1016/S0040-4020(01)92224-0.
- ↑ Won, Mi-Mi (2009). "Analytica Chimica Acta". 631 (1): 54–61.
- ↑ Bates, R. B.; Slagel, R. C. (1962). "Terpenoids. VI. β-Bulnesene, α-guaiene, β-patchoulene, and guaioxide in essential oils". Chemistry & Industry: 1715–1716.
- ↑ Guaiene, Joint FAO/WHO Expert Committee on Food Additives
This article is issued from Wikipedia - version of the 6/6/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.