Greenhouse effect
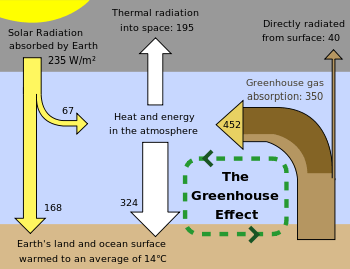

The greenhouse effect is the process by which radiation from a planet's atmosphere warms the planet's surface to a temperature above what it would be without its atmosphere.[1][2]
If a planet's atmosphere contains radiatively active gases (i.e., greenhouse gases) the atmosphere will radiate energy in all directions. Part of this radiation is directed towards the surface, warming it. The downward component of this radiation – that is, the strength of the greenhouse effect – will depend on the atmosphere's temperature and on the amount of greenhouse gases that the atmosphere contains.
On Earth, the atmosphere is warmed by absorption of infrared thermal radiation from the underlying surface, absorption of shorter wavelength radiant energy from the sun, and convective heat fluxes from the surface. Greenhouse gases in the atmosphere radiate energy, some of which is directed to the surface and lower atmosphere. The mechanism that produces this difference between the actual surface temperature and the effective temperature is due to the atmosphere and is known as the greenhouse effect.[3]
Earth’s natural greenhouse effect is critical to supporting life. Human activities, primarily the burning of fossil fuels and clearing of forests, have intensified the natural greenhouse effect, causing global warming.[4]
The mechanism is named after a faulty analogy with the effect of solar radiation passing through glass and warming a greenhouse. The way a greenhouse retains heat is fundamentally different, as a greenhouse works by reducing airflow and retaining warm air inside the structure.[2][5][6]
History
The existence of the greenhouse effect was argued for by Joseph Fourier in 1824. The argument and the evidence was further strengthened by Claude Pouillet in 1827 and 1838, and reasoned from experimental observations by John Tyndall in 1859.[7] The effect was more fully quantified by Svante Arrhenius in 1896.[8] However, the term "greenhouse" was not used to refer to this effect by any of these scientists; the term was first used in this way by Nils Gustaf Ekholm in 1901.[9][10]
In 1917 Alexander Graham Bell wrote "[The unchecked burning of fossil fuels] would have a sort of greenhouse effect", and "The net result is the greenhouse becomes a sort of hot-house."[11][12] Bell went on to also advocate the use of alternate energy sources, such as solar energy.[13]
Mechanism
Earth receives energy from the Sun in the form of ultraviolet, visible, and near-infrared radiation. Of the total amount of solar energy available at the top of the atmosphere, about 26% is reflected to space by the atmosphere and clouds and 19% is absorbed by the atmosphere and clouds. Most of the remaining energy is absorbed at the surface of Earth. Because the Earth's surface is colder than the photosphere of the Sun, it radiates at wavelengths that are much longer than the wavelengths that were absorbed. Most of this thermal radiation is absorbed by the atmosphere, thereby warming it. In addition to the absorption of solar and thermal radiation, the atmosphere further gains heat by sensible and latent heat fluxes from the surface. The atmosphere radiates energy both upwards and downwards; the part radiated downwards is absorbed by the surface of Earth. This leads to a higher equilibrium temperature than if the atmosphere were absent.

An ideal thermally conductive blackbody at the same distance from the Sun as Earth would have a temperature of about 5.3 °C. However, because Earth reflects about 30%[14][15] of the incoming sunlight, this idealized planet's effective temperature (the temperature of a blackbody that would emit the same amount of radiation) would be about −18 °C.[16][17] The surface temperature of this hypothetical planet is 33 °C below Earth's actual surface temperature of approximately 14 °C.[18]
The basic mechanism can be qualified in a number of ways, none of which affect the fundamental process. The atmosphere near the surface is largely opaque to thermal radiation (with important exceptions for "window" bands), and most heat loss from the surface is by sensible heat and latent heat transport. Radiative energy losses become increasingly important higher in the atmosphere, largely because of the decreasing concentration of water vapor, an important greenhouse gas. It is more realistic to think of the greenhouse effect as applying to a "surface" in the mid-troposphere, which is effectively coupled to the surface by a lapse rate. The simple picture also assumes a steady state, but in the real world there are variations due to the diurnal cycle as well as the seasonal cycle and weather disturbances. Solar heating only applies during daytime. During the night, the atmosphere cools somewhat, but not greatly, because its emissivity is low. Diurnal temperature changes decrease with height in the atmosphere.
Within the region where radiative effects are important, the description given by the idealized greenhouse model becomes realistic. Earth's surface, warmed to a temperature around 255 K, radiates long-wavelength, infrared heat in the range of 4–100 μm.[19] At these wavelengths, greenhouse gases that were largely transparent to incoming solar radiation are more absorbent.[19] Each layer of atmosphere with greenhouses gases absorbs some of the heat being radiated upwards from lower layers. It reradiates in all directions, both upwards and downwards; in equilibrium (by definition) the same amount as it has absorbed. This results in more warmth below. Increasing the concentration of the gases increases the amount of absorption and reradiation, and thereby further warms the layers and ultimately the surface below.[17]
Greenhouse gases—including most diatomic gases with two different atoms (such as carbon monoxide, CO) and all gases with three or more atoms—are able to absorb and emit infrared radiation. Though more than 99% of the dry atmosphere is IR transparent (because the main constituents—N2, O2, and Ar—are not able to directly absorb or emit infrared radiation), intermolecular collisions cause the energy absorbed and emitted by the greenhouse gases to be shared with the other, non-IR-active, gases.
Greenhouse gases

By their percentage contribution to the greenhouse effect on Earth the four major gases are:[21][22]
- water vapor, 36–70%
- carbon dioxide, 9–26%
- methane, 4–9%
- ozone, 3–7%
It is not physically realistic to assign a specific percentage to each gas because the absorption and emission bands of the gases overlap (hence the ranges given above). The major non-gas contributor to Earth's greenhouse effect, clouds, also absorb and emit infrared radiation and thus have an effect on the radiative properties of the atmosphere.[22]
Role in climate change
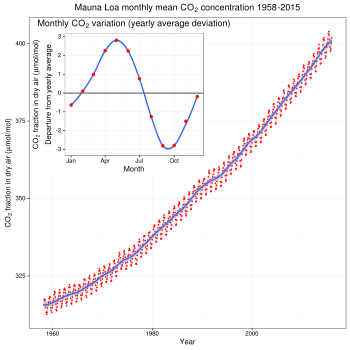
Strengthening of the greenhouse effect through human activities is known as the enhanced (or anthropogenic) greenhouse effect.[23] This increase in radiative forcing from human activity is attributable mainly to increased atmospheric carbon dioxide levels.[24] According to the latest Assessment Report from the Intergovernmental Panel on Climate Change, "atmospheric concentrations of carbon dioxide, methane and nitrous oxide are unprecedented in at least the last 800,000 years. Their effects, together with those of other anthropogenic drivers, have been detected throughout the climate system and are extremely likely to have been the dominant cause of the observed warming since the mid-20th century".[25]
CO2 is produced by fossil fuel burning and other activities such as cement production and tropical deforestation.[26] Measurements of CO2 from the Mauna Loa observatory show that concentrations have increased from about 313 parts per million (ppm)[27] in 1960 to about 389 ppm in 2010. It reached the 400 ppm milestone on May 9, 2013.[28] The current observed amount of CO2 exceeds the geological record maxima (~300 ppm) from ice core data.[29] The effect of combustion-produced carbon dioxide on the global climate, a special case of the greenhouse effect first described in 1896 by Svante Arrhenius, has also been called the Callendar effect.
Over the past 800,000 years,[30] ice core data shows that carbon dioxide has varied from values as low as 180 ppm to the pre-industrial level of 270 ppm.[31] Paleoclimatologists consider variations in carbon dioxide concentration to be a fundamental factor influencing climate variations over this time scale.[32][33]
Real greenhouses
The "greenhouse effect" of the atmosphere is named by analogy to greenhouses which become warmer in sunlight. The explanation given in most sources for the warmer temperature in an actual greenhouse is that incident solar radiation in the visible, long-wavelength ultraviolet, and short-wavelength infrared range of the spectrum passes through the glass roof and walls and is absorbed by the floor, earth, and contents, which become warmer and re-emit the energy as longer-wavelength infrared radiation. Glass and other materials used for greenhouse walls do not transmit infrared radiation, so the infrared cannot escape via radiative transfer. As the structure is not open to the atmosphere, heat also cannot escape via convection, so the temperature inside the greenhouse rises.[34][35] The greenhouse effect, due to infrared-opaque "greenhouse gases" including carbon dioxide and methane instead of glass, also affects Earth as a whole; there is no convective cooling because no significant amount of air escapes from Earth.
However the mechanism by which the atmosphere retains heat—the "greenhouse effect"—is different; a greenhouse is not primarily warmed by the "greenhouse effect".[36] A greenhouse works primarily by allowing sunlight to warm surfaces inside the structure, but then preventing absorbed heat from leaving the structure through convection. The "greenhouse effect" heats Earth because greenhouse gases absorb outgoing radiative energy, heating the atmosphere which then emits radiative energy with some of it going back towards Earth.
A greenhouse is built of any material that passes sunlight, usually glass, or plastic. It mainly warms up because the sun warms the ground and contents inside, which then warms the air in the greenhouse. The air continues to heat up because it is confined within the greenhouse, unlike the environment outside the greenhouse where warm air near the surface rises and mixes with cooler air aloft. This can be demonstrated by opening a small window near the roof of a greenhouse: the temperature will drop considerably. It was demonstrated experimentally (R. W. Wood, 1909) that a "greenhouse" with a cover of rock salt (which is transparent to infrared) heats up an enclosure similarly to one with a glass cover.[6] Thus greenhouses work primarily by preventing convective cooling.[5][37]
More recent quantitative studies suggest that the effect of infrared radiative cooling is not negligibly small, and may have economic implications in a heated greenhouse. Analysis of issues of near-infrared radiation in a greenhouse with screens of a high coefficient of reflection concluded that installation of such screens reduced heat demand by about 8%, and application of dyes to transparent surfaces was suggested. Composite less-reflective glass, or less effective but cheaper anti-reflective coated simple glass, also produced savings.[38]
Bodies other than Earth
In the Solar System, there also greenhouse effects on Mars, Venus, and Titan. The greenhouse effect on Venus is particularly large because its dense atmosphere consisting mainly of carbon dioxide.[39] Titan has an anti-greenhouse effect, in that its atmosphere absorbs solar radiation but is relatively transparent to infrared radiation.[40] Pluto is also colder than would be expected, because evaporation of nitrogen cools it.[41]
A runaway greenhouse effect occurs if positive feedbacks lead to the evaporation of all greenhouse gases into the atmosphere.[42] A runaway greenhouse effect involving carbon dioxide and water vapor is thought to have occurred on Venus.[43]
See also
![]() Global warming portal
Global warming portal
![]() Environment portal
Environment portal
References
- ↑ "Annex II Glossary". Intergovernmental Panel on Climate Change. Retrieved 15 October 2010.
- 1 2 A concise description of the greenhouse effect is given in the Intergovernmental Panel on Climate Change Fourth Assessment Report, "What is the Greenhouse Effect?" FAQ 1.3 - AR4 WGI Chapter 1: Historical Overview of Climate Change Science, IIPCC Fourth Assessment Report, Chapter 1, page 115: "To balance the absorbed incoming [solar] energy, the Earth must, on average, radiate the same amount of energy back to space. Because the Earth is much colder than the Sun, it radiates at much longer wavelengths, primarily in the infrared part of the spectrum (see Figure 1). Much of this thermal radiation emitted by the land and ocean is absorbed by the atmosphere, including clouds, and reradiated back to Earth. This is called the greenhouse effect."
Stephen H. Schneider, in Geosphere-biosphere Interactions and Climate, Lennart O. Bengtsson and Claus U. Hammer, eds., Cambridge University Press, 2001, ISBN 0-521-78238-4, pp. 90-91.
E. Claussen, V. A. Cochran, and D. P. Davis, Climate Change: Science, Strategies, & Solutions, University of Michigan, 2001. p. 373.
A. Allaby and M. Allaby, A Dictionary of Earth Sciences, Oxford University Press, 1999, ISBN 0-19-280079-5, p. 244. - ↑ Vaclav Smil (2003). The Earth's Biosphere: Evolution, Dynamics, and Change. MIT Press. p. 107. ISBN 978-0-262-69298-4.
- ↑ IPCC AR4 WG1 (2007), Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M.; Miller, H.L., eds., Climate Change 2007: The Physical Science Basis, Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, ISBN 978-0-521-88009-1 (pb: 978-0-521-70596-7)
- 1 2 Schroeder, Daniel V. (2000). An introduction to thermal physics. San Francisco, California: Addison-Wesley. pp. 305–7. ISBN 0-321-27779-1.
... this mechanism is called the greenhouse effect, even though most greenhouses depend primarily on a different mechanism (namely, limiting convective cooling).
- 1 2 Wood, R.W. (1909). "Note on the Theory of the Greenhouse". Philosophical Magazine. 17: 319–320. doi:10.1080/14786440208636602.
When exposed to sunlight the temperature rose gradually to 65 °C., the enclosure covered with the salt plate keeping a little ahead of the other because it transmitted the longer waves from the Sun, which were stopped by the glass. In order to eliminate this action the sunlight was first passed through a glass plate." "it is clear that the rock-salt plate is capable of transmitting practically all of it, while the glass plate stops it entirely. This shows us that the loss of temperature of the ground by radiation is very small in comparison to the loss by convection, in other words that we gain very little from the circumstance that the radiation is trapped.
- ↑ John Tyndall, Heat considered as a Mode of Motion (500 pages; year 1863, 1873)
- ↑ Isaac M. Held; Brian J. Soden (Nov 2000). "Water Vapor Feedback and Global Warming". Annual Review of Energy and the Environment. Annual Reviews. 25: 441–475. doi:10.1146/annurev.energy.25.1.441.
- ↑ Easterbrook, Steve. "Who first coined the term "Greenhouse Effect"?". Serendipity. Retrieved 11 November 2015.
- ↑ Ekholm N (1901). "On The Variations Of The Climate Of The Geological And Historical Past And Their Causes". Quarterly Journal of the Royal Meteorological Society. 27 (117): 1–62. doi:10.1002/qj.49702711702.
- ↑ Surtees, Lawrence (2005). "Bell, Alexander Graham". In Cook, Ramsay; Bélanger, Réal. Dictionary of Canadian Biography. XV (1921–1930) (online ed.). University of Toronto Press.
- ↑ Grosvenor, Edwin S. and Morgan Wesson. Alexander Graham Bell: The Life and Times of the Man Who Invented the Telephone. New York: Harry N. Abrahms, Inc., 1997, p. 274, ISBN 0-8109-4005-1.
- ↑ Grosvenor and Wesson, 1997, p. 269.
- ↑ "NASA Earth Fact Sheet". Nssdc.gsfc.nasa.gov. Retrieved 2010-10-15.
- ↑ "Introduction to Atmospheric Chemistry, by Daniel J. Jacob, Princeton University Press, 1999. Chapter 7, "The Greenhouse Effect"". Acmg.seas.harvard.edu. Retrieved 2010-10-15.
- ↑ "Solar Radiation and the Earth's Energy Balance". Eesc.columbia.edu. Retrieved 2010-10-15.
- 1 2 Intergovernmental Panel on Climate Change Fourth Assessment Report. Chapter 1: Historical overview of climate change science page 97
- ↑ The elusive "absolute surface air temperature," see GISS discussion
- 1 2 Mitchell, John F. B. (1989). "THE "GREENHOUSE" EFFECT AND CLIMATE CHANGE" (PDF). Reviews of Geophysics. American Geophysical Union. 27 (1): 115–139. Bibcode:1989RvGeo..27..115M. doi:10.1029/RG027i001p00115. Retrieved 2008-03-23.
- ↑ "NASA: Climate Forcings and Global Warming". January 14, 2009.
- ↑ "Water vapour: feedback or forcing?". RealClimate. 6 April 2005. Retrieved 2006-05-01.
- 1 2 Kiehl, J. T.; Kevin E. Trenberth (February 1997). "Earth's Annual Global Mean Energy Budget" (PDF). Bulletin of the American Meteorological Society. 78 (2): 197–208. Bibcode:1997BAMS...78..197K. doi:10.1175/1520-0477(1997)078<0197:EAGMEB>2.0.CO;2. ISSN 1520-0477. Archived from the original (PDF) on 2006-03-30. Retrieved 2006-05-01.
- ↑ "Enhanced greenhouse effect — Glossary". Nova. Australian Academy of Scihuman impact on the environment. 2006.
- ↑ "Enhanced Greenhouse Effect". Ace.mmu.ac.uk. Retrieved 2010-10-15.
- ↑ IPCC Fifth Assessment Report Synthesis Report: Summary for Policymakers (p. 4)
- ↑ IPCC Fourth Assessment Report, Working Group I Report "The Physical Science Basis" Chapter 7
- ↑ "Atmospheric Carbon Dioxide – Mauna Loa". NOAA.
- ↑ http://news.nationalgeographic.com/news/energy/2013/05/130510-earth-co2-milestone-400-ppm/
- ↑ Hansen J. (February 2005). "A slippery slope: How much global warming constitutes "dangerous anthropogenic interference"?". Climatic Change. 68 (333): 269–279. doi:10.1007/s10584-005-4135-0.
- ↑ "Deep ice tells long climate story". BBC News. 2006-09-04. Retrieved 2010-05-04.
- ↑ Hileman B (2005-11-28). "Ice Core Record Extended". Chemical & Engineering News. 83 (48): 7.
- ↑ Bowen, Mark; Thin Ice: Unlocking the Secrets of Climate in the World's Highest Mountains; Owl Books, 2005.
- ↑ Temperature change and carbon dioxide change, U.S. National Oceanic and Atmospheric Administration
- ↑ A Dictionary of Physics (6 ed.), Oxford University Press, 2009, ISBN 9780199233991: "greenhouse effect"
- ↑ A Dictionary of Chemistry (6 ed.), edited by John Daintith, Publisher: Oxford University Press, 2008, ISBN 9780199204632: "greenhouse effect"
- ↑ Brian Shmaefsky (2004). Favorite demonstrations for college science: an NSTA Press journals collection. NSTA Press. p. 57. ISBN 978-0-87355-242-4.
- ↑ Oort, Abraham H.; Peixoto, José Pinto (1992). Physics of climate. New York: American Institute of Physics. ISBN 0-88318-711-6.
...the name water vapor-greenhouse effect is actually a misnomer since heating in the usual greenhouse is due to the reduction of convection
- ↑ > ENERGY EFFECTS DURING USING THE GLASS WITH DIFFERENT PROPERTIES IN A HEATED GREENHOUSE, Sławomir Kurpaska, Technical Sciences 17(4), 2014, 351–360
- ↑ McKay, C.; Pollack, J.; Courtin, R. (1991). "The greenhouse and antigreenhouse effects on Titan". Science. 253 (5024): 1118–1121. doi:10.1126/science.11538492. PMID 11538492.
- ↑ "Titan: Greenhouse and Anti-greenhouse :: Astrobiology Magazine - earth science - evolution distribution Origin of life universe - life beyond :: Astrobiology is study of earth". Astrobio.net. Retrieved 2010-10-15.
- ↑ "Pluto Colder Than Expected". SPACE.com. 2006-01-03. Retrieved 2010-10-15.
- ↑ Kasting, James F. (1991). "Runaway and moist greenhouse atmospheres and the evolution of Earth and Venus.". Planetary Sciences: American and Soviet Research/Proceedings from the U.S.-U.S.S.R. Workshop on Planetary Sciences. Commission on Engineering and Technical Systems (CETS). pp. 234–245. Retrieved 2009. Check date values in:
|access-date=(help) - ↑ Rasool, I.; De Bergh, C. (Jun 1970). "The Runaway Greenhouse and the Accumulation of CO2 in the Venus Atmosphere" (PDF). Nature. 226 (5250): 1037–1039. Bibcode:1970Natur.226.1037R. doi:10.1038/2261037a0. ISSN 0028-0836. PMID 16057644.
Further reading
- Businger, Joost Alois; Fleagle, Robert Guthrie (1980). An introduction to atmospheric physics. International geophysics series (2nd ed.). San Diego: Academic. ISBN 0-12-260355-9.
- Henderson-Sellers, Ann; McGuffie, Kendal (2005). A climate modelling primer (3rd ed.). New York: Wiley. ISBN 0-470-85750-1.
- Schelling, Thomas C. (2002). "Greenhouse Effect". In David R. Henderson (ed.). Concise Encyclopedia of Economics (1st ed.). Library of Economics and Liberty. OCLC 317650570, 50016270, 163149563
External links
| Wikiversity has learning materials about Topic:Climate change |
![]() Climate Change at Wikibooks
Climate Change at Wikibooks
![]() Media related to Greenhouse effect at Wikimedia Commons
Media related to Greenhouse effect at Wikimedia Commons
![]() The dictionary definition of greenhouse effect at Wiktionary
The dictionary definition of greenhouse effect at Wiktionary