Gouy balance
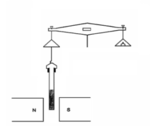
The Gouy balance, invented by Louis Georges Gouy, is a device for measuring the magnetic susceptibility of a sample.
Background
Amongst a wide range of interest in optics, Brownian motion, and experimental physics, Gouy also had a strong intrigue for the phenomena of magnetism. Gouy derived a mathematical expression showing that force is proportional to volume susceptibility for the interaction of material in a uniform magnetic field in 1889. From this derivation, Gouy proposed that balance measurements taken for tubes of material suspended in a magnetic field could evaluate his expression for volume susceptibility. Though Gouy never tested the scientific suggestion himself, this simple and inexpensive method became the foundation for measuring magnetic susceptibility and the blueprint for the Gouy balance.[1]
Procedure
The Gouy balance measures the apparent change in the mass of the sample as it is repelled or attracted by the region of high magnetic field between the poles.[2] Some commercially available balances have a port at their base for this application. In use, a long, cylindrical sample to be tested is suspended from a balance, partially entering between the poles of a magnet. The sample can be in solid or liquid form, and is often placed in a cylindrical container such as a test tube. Solid compounds are generally ground into a fine powder to allow for uniformity amongst the sample.[3] The sample is suspended between the magnetic poles through an attached thread or string.[2] The experimental procedure requires two separate reading to be performed. An initial balance reading is performed on the sample of interest without a magnetic field (m a). A subsequent balance reading is taken with an applied magnetic field (mb). The difference between these two readings relates to the magnetic force on the sample (mb – ma).[2]
Concept
The apparent change in mass from the two balance readings is a result of magnetic force on the sample. The magnetic force is applied across the gradient of a strong and weak magnetic field. A sample with a paramagnetic compound will be pulled down towards the magnetic, and provide a positive difference in apparent mass mb – ma. Diamagnetic compounds can either exhibit no apparent change in weight or a negative change as the sample is slightly repelled by the applied magnetic field.[4] With a paramagnetic sample, the magnetic induction is stronger than the applied field and magnetic susceptibility is positive. A diamagnetic sample has a magnetic induction much weaker than the applied field, and a respective negative magnetic susceptibility.[5] The following mathematical equation relates the apparent change in mass to the volume susceptibility of the sample:
Force = (mb – ma)g = (K2 – K1)AH2 [3]
- mb – ma = apparent difference in mass
- g = gravitational acceleration
- K1 = volume susceptibility of sample
- K2 = volume susceptibility of medium, usually air and of negligible value
- H = applied magnetic field
- A = area of the sample tube
Instrument
In a practical device, the whole assembly of balance and magnet is enclosed in a glass box to ensure that the weight measurement is not affected by air currents. The sample can also be enclosed in a thermostat in order to make measurements at different temperatures.[6] Since it requires a large and powerful electromagnet, the Gouy balance is a stationary instrument permanently set up on a bench.[2] The apparatus is often placed on a marble balance table in a non-ventilated room to minimize the vibrations and disruption from the environment.[5] The stationary magnetic of a Gouy balance is often an electromagnet connected to a power source, since balance recordings with and without the applied magnetic field are required of the procedure.
References
- ↑ Andrea Sella, "Gouy’s Tube", Royal Society of Chemistry, 2010
- 1 2 3 4 Saunderson, A. (1968). "A Permanent Magnet Gouy Balance". Physics Education. 3 (5): 272–273. Bibcode:1968PhyEd...3..272S. doi:10.1088/0031-9120/3/5/007.
- 1 2 Brucacher, L.; Stafford, F. (1962). "Magnetic Susceptibility". J. Chem. Educ. 39: 574. Bibcode:1962JChEd..39..574B. doi:10.1021/ed039p574.
- ↑ Neele Holzenkaempfer, Jesse Gipe "Magnetic Properties of Coordination Complexes", UCDavis ChemWiki
- 1 2 Liang, S.; Harrison, B.; Pagotto, J. (1997). "Determination of the Impregnant Concentrations on ASC Type Charcoal". Defence Research Establishment Ottawa.
- ↑ Earnshaw, Alan (1968). Introduction to Magnetochemistry. Academic Press.p. 89