Ether lipid
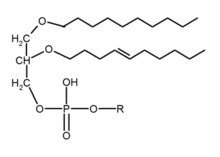
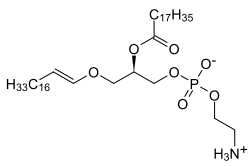
Ether lipids are lipids in which one or more of the carbon atoms on glycerol is bonded to an alkyl chain via an ether linkage, as opposed to the usual ester linkage.
Types
Ether lipids are called plasmalogens (1-O-1'-alkenyl-2-acylglycerophospholipids) if these are glycerol-containing phospholipids with an unsaturated O-(1-alkenyl) (vinyl ether) group at the first position on the glycerol chain.
Platelet-activating factor (PAF) is an ether lipid which has an acetyl group instead of an acyl chain at the second position (SN-2).
Biosynthesis
The formation of the ether bond in mammals requires two enzymes, dihydroxyacetonephosphate acyltransferase (DHAPAT) and alkyldihydroxyacetonephosphate synthase (ADAPS), that reside in the peroxisome.[1] Accordingly, peroxisomal defects often lead to impairment of ether-lipid production.
Monoalkylglycerol ethers (MAGEs) are also generated from 2-acetyl MAGEs (precursors of PAF) by KIAA1363.
Functions
Structural
Plasmalogens as well as some 1-O-alkyl lipids are ubiquitous and sometimes major parts of the cell membranes in mammals and anaerobic bacteria.[2] In archaea, ether lipids are the major polar lipids in the cell envelope and their abundance is one of the major characteristics that separate this group of prokaryotes from the bacteria. In these cells, diphytanylglycerolipids or bipolar macrocyclic tetraethers can form covalently linked 'bilayers'.[3]
Second messenger
Differences between the catabolism of ether glycerophospholipids by specific phospholipases enzymes might be involved in the generation of lipid second messenger systems such as prostaglandins and arachidonic acid that are important in signal transduction.[4] Ether lipids can also act directly in cell signaling, as the platelet-activating factor is an ether lipid signaling molecule that is involved in leukocyte function in the mammalian immune system.[5]
Antioxidant
Another possible function of the plasmalogen ether lipids is as antioxidants, as protective effects against oxidative stress have been demonstrated in cell culture and these lipids might therefore play a role in serum lipoprotein metabolism.[6] This antioxidant activity comes from the enol ether double bond being targeted by a variety of reactive oxygen species.[7]
Synthetic ether lipid analogs
Synthetic ether lipid analogs have cytostatic and cytotoxic properties, probably by disrupting membrane structure and acting as inhibitors of enzymes within signal transmission pathways, such as protein kinase C and phospholipase C.
A toxic ether lipid analogue miltefosine has recently been introduced as an oral treatment for the tropical disease leishmaniasis, which is caused by leishmania, a protozoal parasite with a particularly high ether lipid content in its membranes.[8]
See also
References
- ↑ Hajra AK (1995). "Glycerolipid biosynthesis in peroxisomes (microbodies)". Prog. Lipid Res. 34 (4): 343–64. doi:10.1016/0163-7827(95)00013-5. PMID 8685243.
- ↑ Paltauf F (1994). "Ether lipids in biomembranes". Chem Phys Lipids. 74 (2): 101–39. doi:10.1016/0009-3084(94)90054-X. PMID 7859340.
- ↑ Koga Y, Morii H (2005). "Recent advances in structural research on ether lipids from archaea including comparative and physiological aspects". Biosci Biotechnol Biochem. 69 (11): 2019–34. doi:10.1271/bbb.69.2019. PMID 16306681.
- ↑ Spector A, Yorek M (1 September 1985). "Membrane lipid composition and cellular function". J Lipid Res. 26 (9): 1015–35. PMID 3906008.
- ↑ Demopoulos C, Pinckard R, Hanahan D (10 October 1979). "Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators)". J Biol Chem. 254 (19): 9355–8. PMID 489536.
- ↑ Brosche T, Platt D (1998). "The biological significance of plasmalogens in defense against oxidative damage". Exp Gerontol. 33 (5): 363–9. doi:10.1016/S0531-5565(98)00014-X. PMID 9762517.
- ↑ Engelmann B (2004). "Plasmalogens: targets for oxidants and major lipophilic antioxidants". Biochem Soc Trans. 32 (Pt 1): 147–50. doi:10.1042/BST0320147. PMID 14748736.
- ↑ Lux H, Heise N, Klenner T, Hart D, Opperdoes F (2000). "Ether--lipid (alkyl-phospholipid) metabolism and the mechanism of action of ether--lipid analogues in Leishmania". Mol Biochem Parasitol. 111 (1): 1–14. doi:10.1016/S0166-6851(00)00278-4. PMID 11087912.
External links
- Ether phospholipids at the US National Library of Medicine Medical Subject Headings (MeSH)