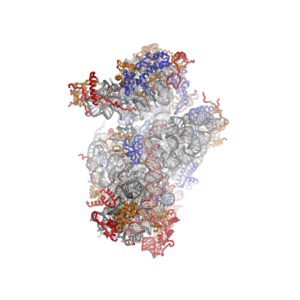
Eukaryotic ribosome (80S)
The ribosome is a large and complex molecular machine that catalyzes the synthesis of proteins, referred to as translation. The ribosome selects aminoacylated transfer RNAs (tRNAs) based on the sequence of a protein-encoding messenger RNA (mRNA) and covalently links the amino acids into a polypeptide chain. Ribosomes from all organisms share a highly conserved catalytic center. However, the ribosomes of eukaryotes (animals, plants, fungi, and many unicellular organisms with a nucleus) are much larger than prokaryotic (bacterial and archaeal) ribosomes and subject to more complex regulation and biogenesis pathways.[1][2] Eukaryotic ribosomes are also known as 80S ribosomes, referring to their sedimentation coefficients in Svedberg units, because they sediment more slowly than the prokaryotic (70S) ribosomes. Eukaryotic ribosomes have two unequal subunits, designated small subunit (40S) and large subunit (60S) according to their sedimentation coefficients. Both subunits contain dozens of ribosomal proteins arranged on a scaffold composed of ribosomal RNA (rRNA). The small subunit monitors the complementarity between tRNA anticodon and mRNA, while the large subunit catalyzes peptide bond formation.
Composition
Compared to their prokaryotic homologs, many of the eukaryotic ribosomal proteins are enlarged by insertions or extensions to the conserved core. Furthermore, several additional proteins are found in the small and large subunits of eukaryotic ribosomes, which do not have prokaryotic homologs. The 40S subunit contains a 18S ribosomal RNA (abbreviated 18S rRNA), which is homologous to the prokaryotic 16S rRNA. The 60S subunit contains a 28S rRNA that is homologous to the prokaryotic 23S ribosomal RNA. In addition, it contains a 5.8S rRNA that corresponds to the 5' end of the 23S rRNA, and a short 5S rRNA. Both 18S and 28S have multiple insertions to the core rRNA fold of their prokaryotic counterparts, which are called expansion segments. For a detailed list of proteins, including archaeal and bacterial homologs please refer to the separate articles on the 40S and 60S subunits. Recent research suggests heterogeneity in the ribosomal composition, i.e., that the stoichiometry among core ribosomal proteins in wild-type yeast cells and embryonic stem cells depends both on the growth conditions and on the number of ribosomes bound per mRNA.[3]
| Eukaryotic[4] | Bacterial[4] | ||
|---|---|---|---|
| Ribosome | Sedimentation coefficient | 80 S | 70 S |
| Molecular mass | ~3.2×106 Da | ~2.0×106 Da | |
| Diameter | ~250-300 Å | ~200 Å | |
| Large subunit | Sedimentation coefficient | 60 S | 50 S |
| Molecular mass | ~2.0×106 Da | ~1.3×106 Da | |
| Proteins | 47 | 33 | |
| rRNAs |
|
| |
| Small subunit | Sedimentation coefficient | 40 S | 30 S |
| Molecular mass | ~1.2×106 Da | ~0.7×106 Da | |
| Proteins | 32 | 20 | |
| rRNAs |
|
| |
Structure determination
Initial structures of eukaryotic ribosomes were determined by electron microscopy. First 3D structures were obtained at 30-40 Å resolution for yeast[5] and mammalian ribosomes.[6][7] Higher resolution structures of the yeast ribosome by cryo-electron microscopy allowed the identification of protein and RNA structural elements.[8] More recently structures at sub-nanometer resolution were obtained for complexes of ribosomes and factors involved in translation.[9][10][11] After the determination of the first bacterial[12][13][14] and archaeal[15] ribosome structures at atomic resolution in the 1990s, it took another decade until in 2011, high resolution structures of eukaryotic ribosome were obtained by X-ray crystallography, mainly because of the difficulties in obtaining crystals of sufficient quality.[16][17][18] The complete structure of a eukaryotic 40S ribosomal structure in Tetrahymena thermophila was published and described, as well as much about the 40S subunit's interaction with eIF1 during translation initiation.[16] The eukaryotic 60S subunit structure was also determined from T. thermophila in complex with eIF6.[17] The complete structure of the eukaryotic 80S ribosome from the yeast Saccharomyces cerevisiae was obtained by crystallography at 3.0 A resolution.[18] These structures reveal the precise architecture of eukaryote-specific elements, their interaction with the universally conserved core, and all eukaryote-specific bridges between the two ribosomal subunits.
Atomic coordinates (PDB files) and structure factors of the eukaryotic ribosome have been deposited in the Protein Data Bank (PDB) under the following accession codes:
| Complex | Source Organism | Resolution | PDB Identifier[19] |
|---|---|---|---|
| 80S:Stm1 | S. cerevisiae | 3.0 Å | |
| 40S:eIF1 | T. thermophila | 3.9 Å | |
| 60S:eIF6 | T. thermophila | 3.5 Å | |
Architecture
General features
Some general architectural features of the ribosome are conserved across kingdoms:[20] The shape of the small subunit can be subdivided into two large segments, the head and the body. Characteristic features of the body include the left and right feet, the shoulder and the platform. The head features a pointed protrusion reminiscent of a bird's beak. In the characteristic "crown view" of the large subunit, structural landmarks include the central protuberance, the L1-stalk and the P-stalk.[21][22] The majority of the eukaryote-specific RNA and protein elements are found on the solvent-exposed sides of the 40S [16] and 60S[17] subunits. The subunit interface, as well as important functional regions such as the peptidyl transferase center and the decoding site are mostly conserved, with some differences observed in the surrounding regions. In stark contrast to prokaryotic ribosomal proteins, which interact primarily with RNA, the eukaryote-specific protein segments engage in a multitude of protein-protein interactions. Long distance interactions are mediated by eukaryote-specific helical extensions of ribosomal proteins, and several eukaryotic ribosomal proteins jointly to form inter-protein beta-sheets.
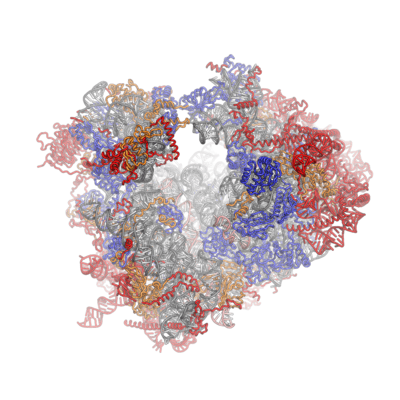
| Crystal structures of the eukaryotic ribosomal subunits from T. thermophila | ||||||||
|---|---|---|---|---|---|---|---|---|
|
The ribosomal RNA core is represented as a grey tube, expansion segments are shown in red. Universally conserved proteins are shown in blue. These proteins have homologs in eukaryotes, archaea and bacteria. Proteins Shared only between eukaryotes and archaea are shown in orange, and proteins specific to eukaryotes are shown in red.
Co-evolution of rRNA and proteins
The structure of the 40S subunit revealed that the eukaryote-specific proteins (rpS7, rpS10, rpS12 and RACK1), as well as numerous eukaryote-specific extensions of proteins, are located on the solvent-exposed side of the small subunit.[16] Here, they participate in the stabilization of rRNA expansion segments. Moreover, the beak of the 40S subunit is remodeled, as rRNA has been replaced by proteins rpS10 and rpS12.[16] As observed for the 40S subunit, all eukaryote-specific proteins of the 60S subunit (RPL6, RPL22, RPL27, RPL28, RPL29 and RPL36) and many extensions are located at the solvent-exposed side, forming an intricate network of interactions with eukaryotic-specific RNA expansion segments. RPL6, RPL27 and RPL29 mediate contacts between the ES sets ES7–ES39, ES31–ES20–ES26 and ES9–ES12, respectively and RPL28 stabilized expansion segment ES7A.[17]
Ubiquitin fusion proteins
In eukaryotes, the small subunit protein rpS27A(S31) and the large subunit protein RPL40 are processed polypeptides, which are translated as fusion proteins carrying N-terminal ubiquitin domains. Both proteins are located next to important functional centers of the ribosome: The uncleaved ubiquitin domains of rpS27A(S31) and RPL40 would be positioned in the decoding site and near the translation factor binding site, respectively. These positions suggest that proteolytic cleavage is an essential step in the production of functional ribosomes.[16][17] Indeed, mutations of the linker between the core of rpS27A(S31) and the ubiquitin domain are lethal in yeast.[23]
Active site
Comparisons between bacterial, archaeal and eukaryotic ribosome structures reveal a very high degree of conservation in the active site -- aka the peptidyl transferase center (PTC) -- region. None of the eukaryote-specific protein elements is close enough to directly participate in catalysis.[17] However, RPL29 projects to within 18Å of the active site in T. thermophila, and eukaryote-specific extensions interlink several proteins in the vicinity of the PTC of the 60S subunit,[17][21] while the corresponding 50S proteins are singular entities.[15]
Intersubunit bridges
Contacts across the two ribosomal subunits are known as intersubunit bridges. In the eukaryotic ribosome, additional contacts are made by 60S expansion segments and proteins.[24] Specifically, the C-terminal extension of the 60S protein RPL19 interacts with ES6E of the 40S rRNA, and the C-terminal extension of the 60S protein RPL24 interacts with 40S rpS6 and rRNA helix h10. Moreover, the 60S expansion segments ES31 and ES41 interact with rpS3A(S1) and rpS8 of the 40S subunit, respectively, and the basic 25-amino-acid peptide RPL41 is positioned at the subunit interface in the 80S ribosome, interacting with rRNA elements of both subunits.[21][24]
Ribosomal proteins with roles in signaling
Two 40S ribosomal proteins (RACK1 and rpS6) have been implicated in signaling: RACK1, the receptor of activated protein kinase C (PKC), is an integral component of the eukaryotic ribosome and is located at the back of the head, in proximity of rpS17.[16] It links signal-transduction pathways directly to the ribosome (reviewed in [25]). Ribosomal protein rpS6 is located at the right foot of the 40S subunit [16] and is phosphorylated in response to mammalian target of rapamycin (mTOR) signaling.[26]
Functional aspects
Translation initiation
Protein synthesis is primarily regulated at the stage of translation initiation. In eukaryotes, the canonical initiation pathway requires at least 12 protein initiation factors, some of which are themselves large complexes.[27] The structures of the 40S:eIF1 [16] and 60S:eIF6 [17] complexes provide first detailed insights into the atomic interactions between the eukaryotic ribosome and regulatory factors. eIF1 is involved in start codon selection, and eIF6 sterically precludes the joining of subunits. However, structural information on the eukaryotic initiation factors and their interactions with the ribosome is limited and largely derived from homology models or low-resolution analyses.[28] Elucidation of the interactions between the eukaryotic ribosome and initiation factors at an atomic level is essential for a mechanistic understanding of the regulatory processes, but represents a significant technical challenge, because of the inherent dynamics and flexibility of the initiation complexes. The first structure of the mammalian pre initiation complex was done by cryo-electron microscopy.[29] Other structures of initiation complexes followed soon, driven by cryo-EM technical improvements.[30][31] Those structures will help better understand the process of translation initiation in eukaryotes.
Regulatory roles of ribosomal proteins
Recent evidence suggests that individual proteins of the eukaryotic ribosome may directly contribute to the regulation of translation.[32][33][34] Specifically, mutations in RPL38 lead to developmental abnormalities, but not to global translational alterations.[35] Moreover, the specific defects [36] and the increased cancer susceptibility [37] that result from mutations in ribosomal proteins may support such a regulatory role.
Protein translocation and targeting
To exert their functions in the cell newly synthesized proteins must be targeted to the appropriate location in the cell, which is achieved by protein targeting and translocation systems.[38] The growing polypeptide leaves the ribosome through a narrow tunnel in the large subunit. The region around the exit tunnel of the 60S subunit is very similar to the bacterial and archaeal 50S subunits. Additional elements are restricted to the second tier of proteins around the tunnel exit, possibly by conserved interactions with components of the translocation machinery.[17] The targeting and translocation machinery is much more complex in eukaryotes.[39]
Ribosomal diseases and cancer
Ribosomopathies are congenital human disorders resulting from defects in ribosomal protein or rRNA genes, or other genes whose products are implicated in ribosome biogenesis.[40] Examples include X-linked Dyskeratosis congenita (X-DC),[37] Diamond–Blackfan anemia,[36] Treacher Collins syndrome (TCS) [36][41] and Shwachman–Bodian–Diamond syndrome (SBDS).[40] SBDS is caused by mutations in the SBDS protein that affects its ability to couple GTP hydrolysis by the GTPase EFL1 to the release of eIF6 from the 60S subunit.[42]
Therapeutic opportunities
The ribosome is a prominent drug target and many antibacterials interfere with translation at different stages of the elongation cycle [43] Most clinically relevant translation compounds are inhibitors of bacterial translation, but inhibitors of eukaryotic translation may also hold therapeutic potential for application in cancer or antifungal chemotherapy.[44] Elongation inhibitors show antitumor activity 'in vivo' and 'in vitro'.[45][46][47] One inhibitor of eukaryotic translation elongation is the glutarimide antibiotic cycloheximide (CHX), which was co-crystallized with the eukaryotic 60S subunit [17] and binds in the ribosomal E-site. The structural characterization of the eukaryotic ribosome [16][17][24] enables the use of structure-based methods for the design of novel therapeutics, and allows the structural differences to the bacterial ribosome to be exploited, improving the selectivity and drug and therefore reducing adverse effects.
References
- ↑ "Difference Between 70S Ribosomes and 80S Ribosomes, RNA, Micromolecules". www.microbiologyprocedure.com. Retrieved 2009-08-06.
- ↑ "80S Ribosomes, Eukaryotic Ribosomes, Prokaryotic Ribosomes, Nucleic Acids, Sedimentation Coefficient". www.microbiologyprocedure.com. Retrieved 2009-08-06.
- ↑ Slavov, Nikolai; Semrau, Stefan; Airoldi, Edoardo; Budnik, Bogdan; van Oudenaarden, Alexander (2015). "Differential Stoichiometry among Core Ribosomal Proteins". Cell Reports. 13 (5): 865–873. doi:10.1016/j.celrep.2015.09.056. ISSN 2211-1247.
- 1 2 Values are based on the ribosomes of T. thermophila (PDB:4A17,4A19) and Thermus thermophilus (PDB 2WDL, 2WDK). The exact size, weight and number of proteins varies from organism to organism.
- ↑ Verschoor, A; Warner, JR; Srivastava, S; Grassucci, RA; Frank, J (Jan 1998). "Three-dimensional structure of the yeast ribosome". Nucleic Acids Res. 26 (2): 655–61. doi:10.1093/nar/26.2.655. PMID 9421530.
- ↑ Verschoor, A; Frank, J (Aug 1990). "Three-dimensional structure of the mammalian cytoplasmic ribosome". J Mol Biol. 214 (3): 737–49. doi:10.1016/0022-2836(90)90289-X. PMID 2388265.
- ↑ "The 80S rat liver ribosome at 25 A resolution by electron cryomicroscopy and angular reconstitution.". Structure. 6 (3): 389–99. Mar 1998. doi:10.1016/s0969-2126(98)00040-9. PMID 9551559.
- ↑ "Structure of the 80S ribosome from Saccharomyces cerevisiae--tRNA-ribosome and subunit-subunit interactions.". Cell. 107 (3): 373–86. Nov 2001. doi:10.1016/s0092-8674(01)00539-6. PMID 11701127.
- ↑ Halic, M; Gartmann, M; Schlenker, O; Mielke, T; Pool, MR; Sinning, I; Beckmann, R (May 2006). "Signal recognition particle receptor exposes the ribosomal translocon binding site". Science. 312 (5774): 745–7. doi:10.1126/science.1124864. PMID 16675701.
- ↑ Becker, T; Bhushan, S; Jarasch, A; Armache, JP; Funes, S; Jossinet, F; Gumbart, J; Mielke, T; Berninghausen, O; Schulten, K; Westhof, E; Gilmore, R; Mandon, EC; Beckmann, R (Dec 2009). "Structure of monomeric yeast and mammalian Sec61 complexes interacting with the translating ribosome". Science. 326 (5958): 1369–73. doi:10.1126/science.1178535. PMID 19933108.
- ↑ Schüler, M; Connell, SR; Lescoute, A; Giesebrecht, J; Dabrowski, M; Schroeer, B; Mielke, T; Penczek, PA; Westhof, E; Spahn, CM (Dec 2006). "Structure of the ribosome-bound cricket paralysis virus IRES RNA". Nat Struct Mol Biol. 13 (12): 1092–6. doi:10.1038/nsmb1177. PMID 17115051.
- ↑ Clemons, WM Jr; May, JL; Wimberly, BT; McCutcheon, JP; Capel, MS; Ramakrishnan, V (Aug 1999). "Structure of a bacterial 30S ribosomal subunit at 5.5 A resolution". Nature. 400 (6747): 833–40. doi:10.1038/23631. PMID 10476960.
- ↑ "X-ray crystal structures of 70S ribosome functional complexes.". Science. 285 (5436): 2095–104. Sep 1999. doi:10.1126/science.285.5436.2095. PMID 10497122.
- ↑ Yusupov, MM; Yusupova, GZ; Baucom, A; Lieberman, K; Earnest, TN; Cate, JH; Noller, HF (May 2001). "Crystal structure of the ribosome at 5.5 A resolution". Science. 292 (5518): 883–96. doi:10.1126/science.1060089. PMID 11283358.
- 1 2 Ban, N; Nissen, P; Hansen, J; Moore, PB; Steitz, TA (Aug 2000). "The complete atomic structure of the large ribosomal subunit at 2.4 A resolution". Science. 289 (5481): 905–20. doi:10.1126/science.289.5481.905. PMID 10937989.
- 1 2 3 4 5 6 7 8 9 10 Rabl, J; Leibundgut, M; Ataide, SF; Haag, A; Ban, N (Feb 2011). "Crystal structure of the eukaryotic 40S ribosomal subunit in complex with initiation factor 1". Science. 331 (6018): 730–6. doi:10.1126/science.1198308. PMID 21205638.
- 1 2 3 4 5 6 7 8 9 10 11 Klinge, S; Voigts-Hoffmann, F; Leibundgut, M; Arpagaus, S; Ban, N (Nov 2011). "Crystal structure of the eukaryotic 60S ribosomal subunit in complex with initiation factor 6". Science. 334 (6058): 941–8. doi:10.1126/science.1211204. PMID 22052974.
- 1 2 Ben-Shem A, Garreau de Loubresse N, Melnikov S, Jenner L, Yusupova G, Yusupov M (February 2011). "The structure of the eukaryotic ribosome at 3.0 Å resolution.". Science. 334 (6062): 1524–1529. doi:10.1126/science.1212642. PMID 22096102.
- ↑ Due to size limitations, ribosome structures are often split into several coordinate files
- ↑ Melnikov, S; Ben-Shem, A; Garreau; de Loubresse, N; Jenner, L; Yusupova, G; Yusupov, M (Jun 2012). "One core, two shells: bacterial and eukaryotic ribosomes". Nat Struct Mol Biol. 19 (6): 560–7. doi:10.1038/nsmb.2313. PMID 22664983.
- 1 2 3 Klinge, S; Voigts-Hoffmann, F; Leibundgut, M; Ban, N (May 2012). "Atomic structures of the eukaryotic ribosome". Trends Biochem Sci. 37 (5): 189–98. doi:10.1016/j.tibs.2012.02.007. PMID 22436288.
- ↑ Jenner, L; Melnikov, S; de Loubresse, NG; Ben-Shem, A; Iskakova, M; Urzhumtsev, A; Meskauskas, A; Dinman, J; Yusupova, G; Yusupov, M (Dec 2012). "Crystal structure of the 80S yeast ribosome". Curr Opin Struct Biol. 22 (6): 759–67. doi:10.1016/j.sbi.2012.07.013. PMID 22884264.
- ↑ Lacombe, T; García-Gómez, JJ; de la Cruz, J; Roser, D; Hurt, E; Linder, P; Kressler, D (Apr 2009). "Linear ubiquitin fusion to Rps31 and its subsequent cleavage are required for the efficient production and functional integrity of 40S ribosomal subunits". Mol Microbiol. 72 (1): 69–84. doi:10.1111/j.1365-2958.2009.06622.x. PMID 19210616.
- 1 2 3 Ben-Shem, A; Garreau; de Loubresse, N; Melnikov, S; Jenner, L; Yusupova, G; Yusupov, M (Dec 2011). "The structure of the eukaryotic ribosome at 3.0 Ã resolution". Science. 334 (6062): 1524–9. doi:10.1126/science.1212642. PMID 22096102.
- ↑ Nilsson, J; Sengupta, J; Frank, J; Nissen, P (Dec 2004). "Regulation of eukaryotic translation by the RACK1 protein: a platform for signalling molecules on the ribosome". EMBO Rep. 5 (12): 1137–41. doi:10.1038/sj.embor.7400291.
- ↑ "The phosphorylated ribosomal protein S7 in Tetrahymena is homologous with mammalian S4 and the phosphorylated residues are located in the C-terminal region. Structural characterization of proteins separated by two-dimensional polyacrylamide gel electrophoresis.". J Biol Chem. 270 (11): 6000–5. Mar 1995. doi:10.1074/jbc.270.11.6000. PMID 7890730.
- ↑ Hinnebusch, AG; Lorsch, JR (Oct 2012). "The mechanism of eukaryotic translation initiation: new insights and challenges". Cold Spring Harb Perspect Biol. 4 (10): a011544. doi:10.1101/cshperspect.a011544. PMID 22815232.
- ↑ Voigts-Hoffmann, F; Klinge, S; Ban, N (Dec 2012). "Structural insights into eukaryotic ribosomes and the initiation of translation". Curr Opin Struct Biol. 22 (6): 768–77. doi:10.1016/j.sbi.2012.07.010. PMID 22889726.
- ↑ Hashem, Y.; Georges, A.; Dhote, V.; Langlois, R.; Liao, H. Y.; Grassucci, R. A.; Frank, J. (2013). "Structure of the mammalian ribosomal 43S preinitiation complex bound to the scanning factor DHX29". Cell. 153 (5): 1108–1119. doi:10.1016/j.cell.2013.04.036.
- ↑ Hashem, Y., Des Georges, A., Dhote, V., Langlois, R., Liao, H. Y., Grassucci, R. A., ... & Frank, J. (2013). Hepatitis-C-virus-like internal ribosome entry sites displace eIF3 to gain access to the 40S subunit. Nature.
- ↑ Fernández, I. S.; Bai, X. C.; Hussain, T.; Kelley, A. C.; Lorsch, J. R.; Ramakrishnan, V.; Scheres, S. H. (2013). "Molecular architecture of a eukaryotic translational initiation complex". Science. 342 (6160): 1240585. doi:10.1126/science.1240585.
- ↑ Gilbert, Wendy V. (2011). "Functional specialization of ribosomes?". Trends in Biochemical Sciences. 36 (3): 127–132. doi:10.1016/j.tibs.2010.12.002. ISSN 0968-0004.
- ↑ Topisirovic, I; Sonenberg, N (Apr 2011). "Translational control by the eukaryotic ribosome". Cell. 145 (3): 333–4. doi:10.1016/j.cell.2011.04.006. PMID 21529706.
- ↑ Preiss, Thomas (2015). "All Ribosomes Are Created Equal. Really?". Trends in Biochemical Sciences. doi:10.1016/j.tibs.2015.11.009. ISSN 0968-0004.
- ↑ Kondrashov, N; Pusic, A; Stumpf, CR; Shimizu, K; Hsieh, AC; Xue, S; Ishijima, J; Shiroishi, T; Barna, M (Apr 2011). "Ribosome-mediated specificity in Hox mRNA translation and vertebrate tissue patterning". Cell. 145 (3): 383–97. doi:10.1016/j.cell.2011.03.028. PMID 21529712.
- 1 2 3 Narla A, Ebert BL. Translational medicine: ribosomopathies. Blood. 2011 Oct 20;118(16):4300-1. doi:10.1182/blood-2011-08-372250
- 1 2 Stumpf, CR; Ruggero, D (Aug 2011). "The cancerous translation apparatus". Curr Opin Genet Dev. 21 (4): 474–83. doi:10.1016/j.gde.2011.03.007. PMC 3481834
 . PMID 21543223.
. PMID 21543223. - ↑ Boehringer, Daniel; Greber, Basil; Ban, Nenad. "Mechanistic insight into co-translational protein processing, folding, targeting, and membrane insertion. Ribosomes, 2011; 405-418. doi:10.1007/978-3-7091-0215-2_32
- ↑ Bohnsack, Markus T.; Schleiff, Enrico. "The evolution of protein targeting and translocation systems". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1803 (10): 1115–1130. doi:10.1016/j.bbamcr.2010.06.005.
- 1 2 Narla, A; Ebert, BL (Apr 2010). "Ribosomopathies: human disorders of ribosome dysfunction". Blood. 115 (16): 3196–205. doi:10.1182/blood-2009-10-178129. PMC 2858486
 . PMID 20194897.
. PMID 20194897. - ↑ Dauwerse, JG; Dixon, J; Seland, S; Ruivenkamp, CA; van Haeringen, A; Hoefsloot, LH; Peters, DJ; Boers, AC; Daumer-Haas, C; Maiwald, R; Zweier, C; Kerr, B; Cobo, AM; Toral, JF; Hoogeboom, AJ; Lohmann, DR; Hehr, U; Dixon, MJ; Breuning, MH; Wieczorek, D (Jan 2011). "Mutations in genes encoding subunits of RNA polymerases I and III cause Treacher Collins syndrome". Nat Genet. 43 (1): 20–2. doi:10.1038/ng.724. PMID 21131976.
- ↑ Finch, AJ; Hilcenko, C; Basse, N; Drynan, LF; Goyenechea, B; Menne, TF; González Fernández, A; Simpson, P; D'Santos, CS; Arends, MJ; Donadieu, J; Bellanné-Chantelot, C; Costanzo, M; Boone, C; McKenzie, AN; Freund, SM; Warren, AJ (May 2011). "Uncoupling of GTP hydrolysis from eIF6 release on the ribosome causes Shwachman-Diamond syndrome". Genes Dev. 25 (9): 917–29. doi:10.1101/gad.623011. PMC 3084026
 . PMID 21536732.
. PMID 21536732. - ↑ Blanchard, SC; Cooperman, BS; Wilson, DN (Jun 2010). "Probing translation with small-molecule inhibitors". Chem Biol. 17 (6): 633–45. doi:10.1016/j.chembiol.2010.06.003. PMID 20609413.
- ↑ Pelletier, J.; Peltz, S.W. (2007). "Therapeutic Opportunities in Translation". Cold Spring Harbor Monograph Archive. 48: 855–895.
- ↑ Schneider-; Poetsch, T.; Usui, T.; et al. (2010a). "Garbled messages and corrupted translations". Nature Methods. 6 (3): 189–198. doi:10.1038/nchembio.326.
- ↑ Schneider; Poetsch, T.; Ju, J.; et al. ", 2010b. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin". Nat Chem Biol. 6 (3): 209–217. doi:10.1038/nchembio.304.
- ↑ Dang, Y.; et al. (2011). "Inhibition of eukaryotic translation elongation by the antitumor natural product Mycalamide B.". RNA. 17 (8): 1578–1588. doi:10.1261/rna.2624511.
Notes
- "EMDB-1067: Ribosomal 80S-eEF2-sordarin complex from S. cerevisiae - EM Navigator". emnavi.protein.osaka-u.ac.jp. Retrieved 2009-08-06.
- Giavalisco P, Wilson D, Kreitler T, et al. (March 2005). "High heterogeneity within the ribosomal proteins of the Arabidopsis thaliana 80S ribosome". Plant Mol. Biol. 57 (4): 577–91. doi:10.1007/s11103-005-0699-3. PMID 15821981.
- "Ribosomes". www.cs.stedwards.edu. Retrieved 2009-08-06.